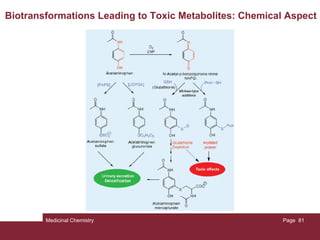
1. The document discusses the processes involved in a drug molecule traveling through the human body from administration to reaching its target site, known as pharmacokinetics.
2. Pharmacokinetics involves absorption of the drug into systemic circulation, distribution of the drug via blood plasma to tissues and organs, and elimination of the drug from the body through biotransformation or excretion.
3. For a drug to have an effect, it must reach the biophase, or site of action, at an appropriate concentration by passing through biological barriers in the body via processes like passive diffusion, carrier-mediated transport, vesicular transport, or paracellular transport between cells.

















![Medicinal Chemistry Page 18
Henderson-Hasselbalch equation
The extent of ionization at a particular pH can be determined
by the Henderson-Hasselbalch equation:
where [RNH2] is the concentration of the free base and [RNH3+] is the
concentration of the ionized amine. Ka is the equilibrium constant for the equilibrium
shown, and the Henderson-Hasselbalch equation can be derived from the
equilibrium constant.
Note that when the concentration of the ionized and unionized amines are identical
(i.e. when [RNH2 = [RNH3+]), the ratio ([RNH2/[RNH3+]) is 1. Since log 1 = 0, the
Henderson-Hasselbalch equation will simplify to pH = pKa.
In other words, when the amine is 50% ionized, pH = pKa.
Therefore, drugs with a pKa of 6-8 are approximately 50% ionized at blood pH (7.4).](https://image.slidesharecdn.com/04-150308100635-conversion-gate01/85/04-pharmacokinetics-1-18-320.jpg)