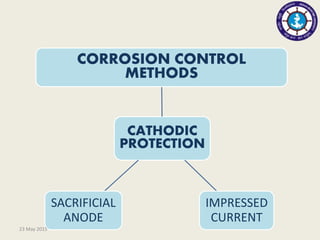
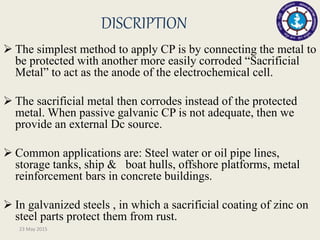
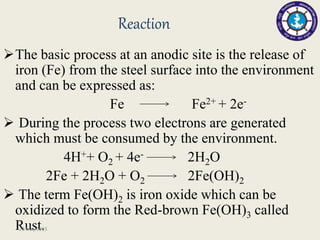
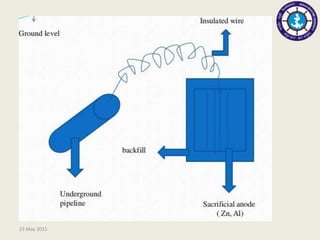
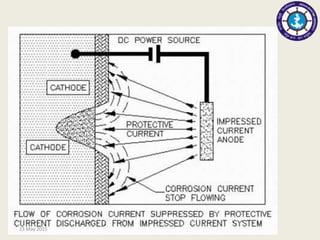
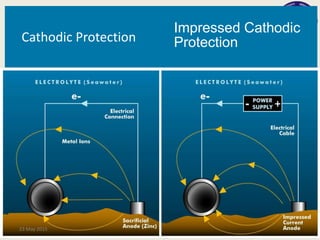
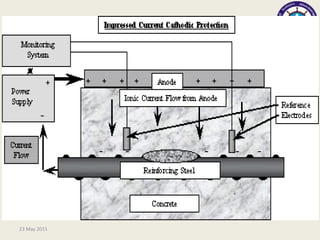
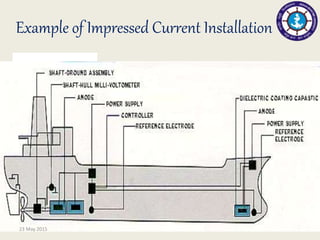
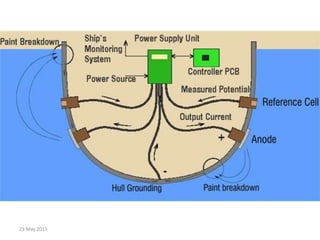
This document discusses cathodic protection, which uses electric current to control corrosion of buried or submerged metal structures. It specifically focuses on impressed current cathodic protection. Impressed current systems use anodes connected to a DC power source to apply an external current and move the metal surface to a negative potential where it is protected from corrosion. Some key applications discussed include pipelines, ships, offshore platforms, and galvanized steel. The document provides a brief history of cathodic protection and describes the basic corrosion reactions and how impressed current systems work to prevent corrosion.