Endothermic and Exothermic Reactions Lesson PowerPoint
- 1. __CH4 + __O2 --> __CO2 + __H2O2 NaCl + 1 BeF2 --> 2 NaF + 1 BeCl2 Na Cl Be F 2 2 2 2 1 1 2 2
- 2. • RED SLIDE: These are notes that are very important and should be recorded in your science journal. Copyright © 2010 Ryan P. Murphy
- 3. -Nice neat notes that are legible and use indents when appropriate. -Example of indent. -Skip a line between topics - -Make visuals clear and well drawn. Label please. Neutron Proton Electron
- 4. • RED SLIDE: These are notes that are very important and should be recorded in your science journal. • BLACK SLIDE: Pay attention, follow directions, complete projects as described and answer required questions neatly. Copyright © 2010 Ryan P. Murphy
- 5. • Keep an eye out for “The-Owl” and raise your hand as soon as you see him. – He will be hiding somewhere in the slideshow Copyright © 2010 Ryan P. Murphy
- 6. “Hoot, Hoot” “Good Luck!” Copyright © 2010 Ryan P. Murphy
- 8. • More Units Available at… Earth Science: The Soil Science and Glaciers Unit, The Geology Topics Unit, The Astronomy Topics Unit, The Weather and Climate Unit, and The River and Water Quality Unit, and The Water Molecule Unit. Physical Science: The Laws of Motion and Machines Unit, The Atoms and Periodic Table Unit, Matter, Energy, and the Environment Unit, and The Science Skills Unit. Life Science: The Infectious Diseases Unit, Cellular Biology Unit, The DNA and Genetics Unit, The Botany Unit, The Taxonomy and Classification Unit, Ecology: Feeding Levels Unit, Ecology: Interactions Unit, Ecology: Abiotic Factors, The Evolution and Natural Selection Unit and The Human Body Systems and Health Topics Unit. Copyright © 2010 Ryan P. Murphy
- 9. • Balancing Chemical Equations.
- 10. • Balancing Chemical Equations. – This is what happens in a chemical reaction
- 11. • Balancing Chemical Equations. – This is what happens in a chemical reaction
- 12. • Balancing Chemical Equations. – This is what happens in a chemical reaction – It describes what you started with…and ended with.
- 13. • Balancing Chemical Equations. – This is what happens in a chemical reaction – It describes what you started with…and ended with.
- 14. • Balancing Chemical Equations. – This is what happens in a chemical reaction – It describes what you started with…and ended with.
- 15. • Balancing Chemical Equations. – This is what happens in a chemical reaction – It describes what you started with…and ended with.
- 16. • Balancing Chemical Equations. – This is what happens in a chemical reaction – It describes what you started with…and ended with.
- 17. • Balancing Chemical Equations. – This is what happens in a chemical reaction – It describes what you started with…and ended with. – It also describes the phases of each (s) (l) (g)
- 18. • Balancing Chemical Equations. – This is what happens in a chemical reaction – It describes what you started with…and ended with. – It also describes the phases of each (s) (l) (g) – It also describes the amount of each.
- 19. • Balancing Chemical Equations. – This is what happens in a chemical reaction – It describes what you started with…and ended with. – It also describes the phases of each (s) (l) (g) – It also describes the amount of each.
- 20. • Balancing Chemical Equations. – This is what happens in a chemical reaction – It describes what you started with…and ended with. – It also describes the phases of each (s) (l) (g) – It also describes the amount of each.
- 21. • Balancing Chemical Equations. – This is what happens in a chemical reaction – It describes what you started with…and ended with. – It also describes the phases of each (s) (l) (g) – It also describes the amount of each.
- 22. • Balancing a chemical equation refers to establishing the mathematical relationship between the quantity of reactants and products.
- 23. • Balancing a chemical equation refers to establishing the mathematical relationship between the quantity of reactants and products. – Reactant: Starting
- 24. • Balancing a chemical equation refers to establishing the mathematical relationship between the quantity of reactants and products. – Reactant: Starting
- 25. • Balancing a chemical equation refers to establishing the mathematical relationship between the quantity of reactants and products. – Reactant: Starting
- 26. • Balancing a chemical equation refers to establishing the mathematical relationship between the quantity of reactants and products. – Reactant: Starting – Products: Ending
- 27. • Balancing a chemical equation refers to establishing the mathematical relationship between the quantity of reactants and products. – Reactant: Starting – Products: Ending
- 28. • Balancing a chemical equation refers to establishing the mathematical relationship between the quantity of reactants and products. – Reactant: Starting – Products: Ending
- 29. • In any physical or chemical change, matter is neither created nor destroyed Copyright © 2010 Ryan P. Murphy
- 30. • In any physical or chemical change, matter is neither created nor destroyed – Matter can be changed from one form to another. Copyright © 2010 Ryan P. Murphy
- 31. • In any physical or chemical change, matter is neither created nor destroyed – Matter can be changed from one form to another. Copyright © 2010 Ryan P. Murphy
- 32. • In any physical or chemical change, matter is neither created nor destroyed – Matter can be changed from one form to another. Copyright © 2010 Ryan P. Murphy
- 33. • In any physical or chemical change, matter is neither created nor destroyed – Matter can be changed from one form to another. Copyright © 2010 Ryan P. Murphy
- 34. • In any physical or chemical change, matter is neither created nor destroyed – Matter can be changed from one form to another. Copyright © 2010 Ryan P. Murphy
- 35. • In any physical or chemical change, matter is neither created nor destroyed – Matter can be changed from one form to another. Copyright © 2010 Ryan P. Murphy
- 36. • In any physical or chemical change, matter is neither created nor destroyed – Matter can be changed from one form to another. Copyright © 2010 Ryan P. Murphy
- 37. • In any physical or chemical change, matter is neither created nor destroyed – Matter can be changed from one form to another. Copyright © 2010 Ryan P. Murphy
- 39. Big Bang All Matter Particles join together
- 40. Big Bang All Matter Particles join together Gravity attracts particles, forms stars, planets Galaxies
- 41. Big Bang All Matter Particles join together Gravity attracts particles, forms stars, planets Galaxies Sun releases particles, photons through nuclear processes
- 42. Big Bang All Matter Particles join together Gravity attracts particles, forms stars, planets Galaxies Sun releases particles, photons through nuclear processes Plants harness Photons to make sugars with available molecules on Earth from formation
- 43. Big Bang All Matter Particles join together Gravity attracts particles, forms stars, planets Galaxies Sun releases particles, photons through nuclear processes Plants harness Photons to make sugars with available molecules on Earth from formation
- 44. Big Bang All Matter Particles join together Gravity attracts particles, forms stars, planets Galaxies Sun releases particles, photons through nuclear processes Plants harness Photons to make sugars with available molecules on Earth from formation Matter from the formation of the planets, sometime after the big bang.
- 45. Big Bang All Matter Particles join together Gravity attracts particles, forms stars, planets Galaxies Sun releases particles, photons through nuclear processes Plants harness Photons to make sugars with available molecules on Earth from formation Matter from the formation of the planets, sometime after the big bang.
- 46. Big Bang All Matter Particles join together Gravity attracts particles, forms stars, planets Galaxies Sun releases particles, photons through nuclear processes Plants harness Photons to make sugars with available molecules on Earth from formation Matter from the formation of the planets, sometime after the big bang.
- 47. Big Bang All Matter Particles join together Gravity attracts particles, forms stars, planets Galaxies Sun releases particles, photons through nuclear processes Plants harness Photons to make sugars with available molecules on Earth from formation Matter from the formation of the planets, sometime after the big bang.
- 48. • Remember the Law Conservation of Mass: Matter cannot be created or destroyed. That means we need to have the same amount of chemicals on each side of the . • For this reason, put a square around the chemical formulas. • Example
- 49. • Remember the Law Conservation of Mass: Matter cannot be created or destroyed. That means we need to have the same amount of chemicals on each side of the . • For this reason, put a square around the chemical formulas. • Example
- 50. • Remember the Law Conservation of Mass: Matter cannot be created or destroyed. That means we need to have the same amount of chemicals on each side of the . • For this reason, put a square around the chemical formulas.
- 51. • Remember the Law Conservation of Mass: Matter cannot be created or destroyed. That means we need to have the same amount of chemicals on each side of the . • For this reason, put a square around the chemical formulas. • Example
- 52. • Begin balancing chemical equations by putting numbers (coefficients) in front of them.
- 53. • Begin balancing chemical equations by putting numbers (coefficients) in front of them. – Example H2O on one side could become 2 H2O
- 54. • Begin balancing chemical equations by putting numbers (coefficients) in front of them. – Example H2O on one side could become 2 H2O – Remember that each side needs to have same number of Hydrogen and Oxygen
- 55. • Begin balancing chemical equations by putting numbers (coefficients) in front of them. – Example H2O on one side could become 2 H2O – Remember that each side needs to have same number of Hydrogen and Oxygen • Note – Don’t change the subscript • Example H2O becomes H3O
- 56. • Begin balancing chemical equations by putting numbers (coefficients) in front of them. – Example H2O on one side could become 2 H2O – Remember that each side needs to have same number of Hydrogen and Oxygen • Note – Don’t change the subscript • Example H2O becomes H3O
- 57. • Balancing Equations Available Sheet. – Complete each equation as we cover it in class.
- 59. • A way to start off the process is to create an inventory of your chemicals.
- 60. • A way to start off the process is to create an inventory of your chemicals. BOXES!!!
- 61. • A way to start off the process is to create an inventory of your chemicals.
- 62. • A way to start off the process is to create an inventory of your chemicals.
- 63. • A way to start off the process is to create an inventory of your chemicals.
- 64. • A way to start off the process is to create an inventory of your chemicals.
- 65. • A way to start off the process is to create an inventory of your chemicals.
- 66. • A way to start off the process is to create an inventory of your chemicals.
- 67. • A way to start off the process is to create an inventory of your chemicals.
- 68. • A way to start off the process is to create an inventory of your chemicals.
- 69. • A way to start off the process is to create an inventory of your chemicals.
- 70. • A way to start off the process is to create an inventory of your chemicals.
- 71. • A way to start off the process is to create an inventory of your chemicals.
- 72. • A way to start off the process is to create an inventory of your chemicals.
- 73. • A way to start off the process is to create an inventory of your chemicals.
- 74. • A way to start off the process is to create an inventory of your chemicals.
- 75. • A way to start off the process is to create an inventory of your chemicals.
- 76. • A way to start off the process is to create an inventory of your chemicals.
- 77. • A way to start off the process is to create an inventory of your chemicals.
- 78. • A way to start off the process is to create an inventory of your chemicals.
- 79. • A way to start off the process is to create an inventory of your chemicals.
- 80. • A way to start off the process is to create an inventory of your chemicals.
- 81. • A way to start off the process is to create an inventory of your chemicals.
- 82. • A way to start off the process is to create an inventory of your chemicals.
- 83. • A way to start off the process is to create an inventory of your chemicals.
- 84. • A way to start off the process is to create an inventory of your chemicals.
- 85. • A way to start off the process is to create an inventory of your chemicals.
- 86. • A way to start off the process is to create an inventory of your chemicals.
- 87. • A way to start off the process is to create an inventory of your chemicals.
- 88. • A way to start off the process is to create an inventory of your chemicals.
- 89. • A way to start off the process is to create an inventory of your chemicals.
- 90. • A way to start off the process is to create an inventory of your chemicals.
- 91. • A way to start off the process is to create an inventory of your chemicals.
- 92. • A way to start off the process is to create an inventory of your chemicals.
- 93. • A way to start off the process is to create an inventory of your chemicals.
- 94. • Now look at the inventory and begin the process of balancing the equation.
- 96. One Sodium
- 97. One Sodium Two Sodium
- 98. One Sodium Two Sodium Let’s add a 2 here and see if it balances. 2
- 99. Did it balance? Are we done? 2
- 100. Did it balance? Are we done? We should do a new inventory chart. 2
- 101. 2
- 102. 2
- 103. 2
- 104. 2 2
- 105. 2 2
- 106. 2 2
- 107. 2 2 2
- 108. 2 2 2
- 109. 2 2 2
- 110. 2 2 2 6
- 111. 2 2 2 6
- 112. 2 2 2 6
- 113. 2 2 2 6 5
- 114. 2 2 2 6 5
- 115. One Sodium Two Sodium Let’s try again and add a 2 to the other side. 2 2
- 116. Does it balance this time?
- 129. This is a balanced equation.
- 130. • It should work most of the time although it can be very tricky. Always keep an inventory chart or it will get all messed up. __CH4 + __O2 --> __CO2 + __H2O__CH4 + __O2 --> __CO2 + __H2O C H O
- 131. • It should work most of the time although it can be very tricky. Always keep an inventory chart or it will get all messed up. Try to balance… __CH4 + __O2 --> __CO2 + __H2O__CH4 + __O2 --> __CO2 + __H2O C H O
- 133. • It should work most of the time although it can be very tricky. Always keep an inventory chart or it will get all messed up. Try to balance… __CH4 + __O2 --> __CO2 + __H2O__CH4 + __O2 --> __CO2 + __H2O C H O
- 134. • It should work most of the time although it can be very tricky. Always keep an inventory chart or it will get all messed up. Try to balance… BOXES! __CH4 + __O2 --> __CO2 + __H2O__CH4 + __O2 --> __CO2 + __H2O C H O
- 135. • It should work most of the time although it can be very tricky. Always keep an inventory chart or it will get all messed up. Try to balance… __CH4 + __O2 --> __CO2 + __H2O__CH4 + __O2 --> __CO2 + __H2O C H O
- 136. • It should work most of the time although it can be very tricky. Always keep an inventory chart or it will get all messed up. Try to balance… __CH4 + __O2 --> __CO2 + __H2O__CH4 + __O2 --> __CO2 + __H2O C H O 1 1
- 137. • It should work most of the time although it can be very tricky. Always keep an inventory chart or it will get all messed up. Try to balance… __CH4 + __O2 --> __CO2 + __H2O__CH4 + __O2 --> __CO2 + __H2O C H O 1 1
- 138. • It should work most of the time although it can be very tricky. Always keep an inventory chart or it will get all messed up. Try to balance… __CH4 + __O2 --> __CO2 + __H2O__CH4 + __O2 --> __CO2 + __H2O C H O 1 1 4 2
- 139. • It should work most of the time although it can be very tricky. Always keep an inventory chart or it will get all messed up. Try to balance… __CH4 + __O2 --> __CO2 + __H2O__CH4 + __O2 --> __CO2 + __H2O C H O 1 1 4 2
- 140. • It should work most of the time although it can be very tricky. Always keep an inventory chart or it will get all messed up. Try to balance… __CH4 + __O2 --> __CO2 + __H2O__CH4 + __O2 --> __CO2 + __H2O C H O 1 1 4 2 2 3
- 141. __CH4 + __O2 --> __CO2 + __H2O__CH4 + __O2 --> __CO2 + __H2O C H O 1 1 4 2 2 3
- 142. __CH4 + __O2 --> __CO2 + __H2O__CH4 + __O2 --> __CO2 + __H2O C H O 1 1 4 2 2 3 What should we put to equal 4?
- 143. • See if this is right? __CH4 + __O2 --> __CO2 + __H2O__CH4 + __O2 --> __CO2 + __H2O C H O
- 144. • Answer: Incorrect – The inventory does not match. __CH4 + __O2 --> __CO2 + __H2O1 CH4 + 2 O2 --> 2 CO2 + 2 H2O C H O 1 2 4 4 4 5
- 145. • Answer: See if this is right? __CH4 + __O2 --> __CO2 + __H2O1 CH4 + 2 O2 --> 1 CO2 + 2 H2O C H O
- 146. • Answer: See if this is right? __CH4 + __O2 --> __CO2 + __H2O1 CH4 + 2 O2 --> 1 CO2 + 2 H2O C H O
- 147. • Answer: See if this is right? • Answer: Yes, A balanced equation __CH4 + __O2 --> __CO2 + __H2O1 CH4 + 2 O2 --> 1 CO2 + 2 H2O C H O 1 1 4 4 4 4
- 148. • What’s this famous equation? ___CO2 + ___H2O + light energy = __C6H12O6 + __O2
- 149. • What’s this famous equation? ___CO2 + ___H2O + light energy = __C6H12O6 + __O2
- 150. • What’s this famous equation? • Can you balance it? ___CO2 + ___H2O + light energy = __C6H12O6 + __O2 ___CO2 + ___H2O = __C6H12O6 + __O2 Element Before After
- 151. • What’s this famous equation? • Can you balance it? ___CO2 + ___H2O + light energy = __C6H12O6 + __O2 ___CO2 + ___H2O = __C6H12O6 + __O2 Element Before After 1 6 2 122 3 8
- 152. • What’s this famous equation? • Does this balance? ___CO2 + ___H2O + light energy = __C6H12O6 + __O2 ___CO2 + ___H2O = __C6H12O6 + __O2 Element Before After
- 153. • What’s this famous equation? • Does this balance? ___CO2 + ___H2O + light energy = __C6H12O6 + __O2 ___CO2 + ___H2O = __C6H12O6 + __O2 Element Before After 6 6 12 12 18 18
- 154. • What’s this famous equation? • Does this balance? ___CO2 + ___H2O + light energy = __C6H12O6 + __O2 ___CO2 + ___H2O = __C6H12O6 + __O2 Element Before After 6 6 12 12 18 18
- 155. • Which of the following equations is the correct equation for photosynthesis? • A) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • B) 6CO2 + 6H2O + sugar = C6H12O6 + 6O2 • C) 6CO2 + 6CO2 + light energy = C6H12O6 + 6H2O • D) 6CO2 + 6H2 + light energy = C6H12O6 + 6H2O • E) 6CO2 + 6H2O + light energy = C6H12O6 + 6O2 • F) 6CO2 + 6H2O + light energy = C6H2O6 + 6O2 • G) 6CO2 + 6H2O + sugar = C6H12O6 + 6O2 • H) 6CO2 + 6H2O + light energy = C6H12O6 + 6CO2 • I) 6CO2 + H2O + light energy = C6H12O6 + 6O2 • J) C6H12O6 = 6CO2 + 6H2O + light energy + 6O2 Copyright © 2010 Ryan P. Murphy
- 156. • Which of the following equations is the correct equation for photosynthesis? • A) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • B) 6CO2 + 6H2O + sugar = C6H12O6 + 6O2 • C) 6CO2 + 6CO2 + light energy = C6H12O6 + 6H2O • D) 6CO2 + 6H2 + light energy = C6H12O6 + 6H2O • E) 6CO2 + 6H2O + light energy = C6H12O6 + 6O2 • F) 6CO2 + 6H2O + light energy = C6H2O6 + 6O2 • G) 6CO2 + 6H2O + sugar = C6H12O6 + 6O2 • H) 6CO2 + 6H2O + light energy = C6H12O6 + 6CO2 • I) 6CO2 + H2O + light energy = C6H12O6 + 6O2 • J) C6H12O6 = 6CO2 + 6H2O + light energy + 6O2 Copyright © 2010 Ryan P. Murphy
- 157. • Find the Photosynthesis equation. • A) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • B) 6CO2 + 6H2O + sugar = C6H12O6 + 6O2 • C) 6CO2 + 6O2 + light energy = C6H12O6 + 6H2O • D) 6CO2 + 12H2O + light energy = C6H12O6 + 6H2O • E) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • F) 6CO2 + 6H2O + light energy = C6H2O6 + 6O2 • G) 6CO2 + 6H2 + light energy = C6H12O6 + 6O2 • H) 6CO2 + 6H2O + light energy = C6H12O6 + 6O6 • I) 6CO2 + 6H6O + light energy = C6H12O6 + 6O2 • J) 6CO2 + 6H2O + light energy = CH12O6 + 6CO2 • K) CO2 + 6H2O2 + light energy = CH12O6 + 6O2 • L) 6CO2 + 6H2O + no energy = C6H12O6 + 6O2 • M) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • N) 6CO2 + 6H2O + sugar = C6H12O6 + 6O2 • O) 6CO2 + 6O2 + light energy = C6H12O6 + 6H2O • P) 6CO2 + 12H2O + light energy = C6H12O6 + 6H2O • Q) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • R) 6CO2 + 6H2O + light energy = C6H2O6 + 6O2 • S) 6CO2 + 6H2O + light energy = C6H12O6 + 6O2 • T) 6CO2 + 6H2O + light energy = C6H12O6 + 6O6 • U) 6CO2 + 6H6O + light energy = C6H12O6 + 6O2 • V) 6CO2 + 6H2O + light energy = CH12O6 + 6CO2 • W) CO2 + 6H2O2 + light energy = CH12O6 + 6O2 • X) 6CO2 + 6H2O + no energy = C6H12O6 + 6O2 • Y) 6CO2 + 6H2 + light energy = C6H12O6 + 6O2 • Z) 6CO2 + 6H2O + light energy = C6H12O6 + 6O6 Copyright © 2010 Ryan P. Murphy
- 158. • Find the Photosynthesis equation. • A) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • B) 6CO2 + 6H2O + sugar = C6H12O6 + 6O2 • C) 6CO2 + 6O2 + light energy = C6H12O6 + 6H2O • D) 6CO2 + 12H2O + light energy = C6H12O6 + 6H2O • E) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • F) 6CO2 + 6H2O + light energy = C6H2O6 + 6O2 • G) 6CO2 + 6H2 + light energy = C6H12O6 + 6O2 • H) 6CO2 + 6H2O + light energy = C6H12O6 + 6O6 • I) 6CO2 + 6H6O + light energy = C6H12O6 + 6O2 • J) 6CO2 + 6H2O + light energy = CH12O6 + 6CO2 • K) CO2 + 6H2O2 + light energy = CH12O6 + 6O2 • L) 6CO2 + 6H2O + no energy = C6H12O6 + 6O2 • M) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • N) 6CO2 + 6H2O + sugar = C6H12O6 + 6O2 • O) 6CO2 + 6O2 + light energy = C6H12O6 + 6H2O • P) 6CO2 + 12H2O + light energy = C6H12O6 + 6H2O • Q) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • R) 6CO2 + 6H2O + light energy = C6H2O6 + 6O2 • S) 6CO2 + 6H2O + light energy = C6H12O6 + 6O2 • T) 6CO2 + 6H2O + light energy = C6H12O6 + 6O6 • U) 6CO2 + 6H6O + light energy = C6H12O6 + 6O2 • V) 6CO2 + 6H2O + light energy = CH12O6 + 6CO2 • W) CO2 + 6H2O2 + light energy = CH12O6 + 6O2 • X) 6CO2 + 6H2O + no energy = C6H12O6 + 6O2 • Y) 6CO2 + 6H2 + light energy = C6H12O6 + 6O2 • Z) 6CO2 + 6H2O + light energy = C6H12O6 + 6O6 Copyright © 2010 Ryan P. Murphy
- 159. • What’s this famous equation? ___C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O.
- 160. • What’s this famous equation? • Can you balance it? ___C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O.
- 161. • What’s this famous equation? • Can you balance it? ___C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O.
- 162. • What’s this famous equation? • Can you balance it? ___C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. ___+C6H12O6 + __O2 = Released energy + __CO2 + __H2O Element Before After
- 163. • What’s this famous equation? • Can you balance it? ___C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. ___+C6H12O6 + __O2 = Released energy + __CO2 + __H2O Element Before After 6 1 12 2 8 3
- 164. • What’s this famous equation? • Will this balance? ___C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. ___+C6H12O6 + __O2 = Released energy + __CO2 + __H2O Element Before After
- 165. • What’s this famous equation? • Will this balance? ___C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. ___+C6H12O6 + __O2 = Released energy + __CO2 + __H2O Element Before After 6 6 12 12 18 18
- 166. • What’s this famous equation? • Will this balance? ___C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. ___+C6H12O6 + __O2 = Released energy + __CO2 + __H2O Element Before After 6 6 12 12 18 18
- 167. • Which of the following equations is the correct one for the respiration equation? • A) C6H12O6 + 6H2O = Released energy + 6CO2 + 6H2O. • B) C6H12O6 + O2 = Released energy + C6H12O6 + 6CO2 + 6H2O. • C) C6H12O6 + 6O2 = Released energy + 6O2 + 6H2O. • D) C12H6O6 + 6O2 = Released energy + 6CO2 + 6H2O. • E) C6H12O6 + 6CO2 = Released energy + 6O2 + 6H2O. • F) C6H12O6 + 6CO2 = Released energy + 6CO2 + 6H2O. • G) C6H12O6 + 6O2 = Released energy + 6CO2 + 62O. • H) C6H12O6 + 6O2 = Light Energy + 6CO2 + 6H2O. • I) C6H12O6 + 6O2 = Released energy + 6O2 + 6H2O. • J) C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. • K) C6H12O6 + 16O2 = Released energy + 6O2 + 6H2O. • L) Released energy + 6CO2 + 6H2O C6H12O6 + 6O2 = Copyright © 2010 Ryan P. Murphy
- 168. • Answer! Which of the following equations is the correct one for the respiration equation? • A) C6H12O6 + 6H2O = Released energy + 6CO2 + 6H2O. • B) C6H12O6 + O2 = Released energy + C6H12O6 + 6CO2 + 6H2O. • C) C6H12O6 + 6O2 = Released energy + 6O2 + 6H2O. • D) C12H6O6 + 6O2 = Released energy + 6CO2 + 6H2O. • E) C6H12O6 + 6CO2 = Released energy + 6O2 + 6H2O. • F) C6H12O6 + 6CO2 = Released energy + 6CO2 + 6H2O. • G) C6H12O6 + 6O2 = Released energy + 6CO2 + 62O. • H) C6H12O6 + 6O2 = Light Energy + 6CO2 + 6H2O. • I) C6H12O6 + 6O2 = Released energy + 6O2 + 6H2O. • J) C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. • K) C6H12O6 + 16O2 = Released energy + 6O2 + 6H2O. • L) Released energy + 6CO2 + 6H2O C6H12O6 + 6O2 = Copyright © 2010 Ryan P. Murphy
- 170. • Which one is the equation for photosynthesis, and which one is the equation for respiration? • A) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • B) C6H12O6 + O2 = Released energy + 6CO2 + 6H2O. • C) 6CO2 + 6O2 + light energy = C6H12O6 + 6H2O • D) 6CO2 + 12H2O + light energy = C6H12O6 + 6H2O • E) C6H12O6 + 6O2 = Released energy + 6O2 + H2O. • F) 6CO2 + 6H2O + light energy = C6H12O6 + 6O2 • G) 6CO2 + 6H2O + light energy = C6H12O6 + 6CO2 • H) C6H12O6 + 6CO2 = Released energy + 6CO2 + 6H2O. • I) 6CO2 + 6H6O + light energy = C6H12O6 + 6O2 • J) 6CO2 + 6H2O + light energy = CH12O6 + 6CO2 • K) C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. • L) 6CO2 + 6H2O + no energy = C6H12O6 + 6O2 • M) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • N.) C6H12O6 + 6O2 = light energy + 6CO2 + 6H2O. Copyright © 2010 Ryan P. Murphy
- 171. • Which one is the equation for photosynthesis, and which one is the equation for respiration? • A) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • B) C6H12O6 + O2 = Released energy + 6CO2 + 6H2O. • C) 6CO2 + 6O2 + light energy = C6H12O6 + 6H2O • D) 6CO2 + 12H2O + light energy = C6H12O6 + 6H2O • E) C6H12O6 + 6O2 = Released energy + 6O2 + H2O. • F) 6CO2 + 6H2O + light energy = C6H12O6 + 6O2 • G) 6CO2 + 6H2O + light energy = C6H12O6 + 6CO2 • H) C6H12O6 + 6CO2 = Released energy + 6CO2 + 6H2O. • I) 6CO2 + 6H6O + light energy = C6H12O6 + 6O2 • J) 6CO2 + 6H2O + light energy = CH12O6 + 6CO2 • K) C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. • L) 6CO2 + 6H2O + no energy = C6H12O6 + 6O2 • M) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • N.) C6H12O6 + 6O2 = light energy + 6CO2 + 6H2O. Copyright © 2010 Ryan P. Murphy
- 172. • Which one is the equation for photosynthesis, and which one is the equation for respiration? • A) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • B) C6H12O6 + O2 = Released energy + 6CO2 + 6H2O. • C) 6CO2 + 6O2 + light energy = C6H12O6 + 6H2O • D) 6CO2 + 12H2O + light energy = C6H12O6 + 6H2O • E) C6H12O6 + 6O2 = Released energy + 6O2 + H2O. • F) 6CO2 + 6H2O + light energy = C6H12O6 + 6O2 • G) 6CO2 + 6H2O + light energy = C6H12O6 + 6CO2 • H) C6H12O6 + 6CO2 = Released energy + 6CO2 + 6H2O. • I) 6CO2 + 6H6O + light energy = C6H12O6 + 6O2 • J) 6CO2 + 6H2O + light energy = CH12O6 + 6CO2 • K) C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. • L) 6CO2 + 6H2O + no energy = C6H12O6 + 6O2 • M) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • N.) C6H12O6 + 6O2 = light energy + 6CO2 + 6H2O. Copyright © 2010 Ryan P. Murphy
- 173. • Which one is the equation for photosynthesis, and which one is the equation for respiration? • A) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • B) C6H12O6 + O2 = Released energy + 6CO2 + 6H2O. • C) 6CO2 + 6O2 + light energy = C6H12O6 + 6H2O • D) 6CO2 + 12H2O + light energy = C6H12O6 + 6H2O • E) C6H12O6 + 6O2 = Released energy + 6O2 + H2O. • F) 6CO2 + 6H2O + light energy = C6H12O6 + 6O2 • G) 6CO2 + 6H2O + light energy = C6H12O6 + 6CO2 • H) C6H12O6 + 6CO2 = Released energy + 6CO2 + 6H2O. • I) 6CO2 + 6H6O + light energy = C6H12O6 + 6O2 • J) 6CO2 + 6H2O + light energy = CH12O6 + 6CO2 • K) C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. • L) 6CO2 + 6H2O + no energy = C6H12O6 + 6O2 • M) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • N.) C6H12O6 + 6O2 = light energy + 6CO2 + 6H2O. Copyright © 2010 Ryan P. Murphy
- 174. • Which one is the equation for photosynthesis, and which one is the equation for respiration? • A) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • B) C6H12O6 + O2 = Released energy + 6CO2 + 6H2O. • C) 6CO2 + 6O2 + light energy = C6H12O6 + 6H2O • D) 6CO2 + 12H2O + light energy = C6H12O6 + 6H2O • E) C6H12O6 + 6O2 = Released energy + 6O2 + H2O. • F) 6CO2 + 6H2O + light energy = C6H12O6 + 6O2 • G) 6CO2 + 6H2O + light energy = C6H12O6 + 6CO2 • H) C6H12O6 + 6CO2 = Released energy + 6CO2 + 6H2O. • I) 6CO2 + 6H6O + light energy = C6H12O6 + 6O2 • J) 6CO2 + 6H2O + light energy = CH12O6 + 6CO2 • K) C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. • L) 6CO2 + 6H2O + no energy = C6H12O6 + 6O2 • M) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • N.) C6H12O6 + 6O2 = light energy + 6CO2 + 6H2O. Copyright © 2010 Ryan P. Murphy
- 175. • Which one is the equation for photosynthesis, and which one is the equation for respiration? • A) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • B) C6H12O6 + O2 = Released energy + 6CO2 + 6H2O. • C) 6CO2 + 6O2 + light energy = C6H12O6 + 6H2O • D) 6CO2 + 12H2O + light energy = C6H12O6 + 6H2O • E) C6H12O6 + 6O2 = Released energy + 6O2 + H2O. • F) 6CO2 + 6H2O + light energy = C6H12O6 + 6O2 • G) 6CO2 + 6H2O + light energy = C6H12O6 + 6CO2 • H) C6H12O6 + 6CO2 = Released energy + 6CO2 + 6H2O. • I) 6CO2 + 6H6O + light energy = C6H12O6 + 6O2 • J) 6CO2 + 6H2O + light energy = CH12O6 + 6CO2 • K) C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. • L) 6CO2 + 6H2O + no energy = C6H12O6 + 6O2 • M) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • N.) C6H12O6 + 6O2 = light energy + 6CO2 + 6H2O. Copyright © 2010 Ryan P. Murphy
- 176. • Which one is the equation for photosynthesis, and which one is the equation for respiration? • A) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • B) C6H12O6 + O2 = Released energy + 6CO2 + 6H2O. • C) 6CO2 + 6O2 + light energy = C6H12O6 + 6H2O • D) 6CO2 + 12H2O + light energy = C6H12O6 + 6H2O • E) C6H12O6 + 6O2 = Released energy + 6O2 + H2O. • F) 6CO2 + 6H2O + light energy = C6H12O6 + 6O2 • G) 6CO2 + 6H2O + light energy = C6H12O6 + 6CO2 • H) C6H12O6 + 6CO2 = Released energy + 6CO2 + 6H2O. • I) 6CO2 + 6H6O + light energy = C6H12O6 + 6O2 • J) 6CO2 + 6H2O + light energy = CH12O6 + 6CO2 • K) C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. • L) 6CO2 + 6H2O + no energy = C6H12O6 + 6O2 • M) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • N.) C6H12O6 + 6O2 = light energy + 6CO2 + 6H2O. Copyright © 2010 Ryan P. Murphy
- 177. • Which one is the equation for photosynthesis, and which one is the equation for respiration? • A) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • B) C6H12O6 + O2 = Released energy + 6CO2 + 6H2O. • C) 6CO2 + 6O2 + light energy = C6H12O6 + 6H2O • D) 6CO2 + 12H2O + light energy = C6H12O6 + 6H2O • E) C6H12O6 + 6O2 = Released energy + 6O2 + H2O. • F) 6CO2 + 6H2O + light energy = C6H12O6 + 6O2 • G) 6CO2 + 6H2O + light energy = C6H12O6 + 6CO2 • H) C6H12O6 + 6CO2 = Released energy + 6CO2 + 6H2O. • I) 6CO2 + 6H6O + light energy = C6H12O6 + 6O2 • J) 6CO2 + 6H2O + light energy = CH12O6 + 6CO2 • K) C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. • L) 6CO2 + 6H2O + no energy = C6H12O6 + 6O2 • M) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • N.) C6H12O6 + 6O2 = light energy + 6CO2 + 6H2O. Copyright © 2010 Ryan P. Murphy
- 178. • Which one is the equation for photosynthesis, and which one is the equation for respiration? • A) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • B) C6H12O6 + O2 = Released energy + 6CO2 + 6H2O. • C) 6CO2 + 6O2 + light energy = C6H12O6 + 6H2O • D) 6CO2 + 12H2O + light energy = C6H12O6 + 6H2O • E) C6H12O6 + 6O2 = Released energy + 6O2 + H2O. • F) 6CO2 + 6H2O + light energy = C6H12O6 + 6O2 • G) 6CO2 + 6H2O + light energy = C6H12O6 + 6CO2 • H) C6H12O6 + 6CO2 = Released energy + 6CO2 + 6H2O. • I) 6CO2 + 6H6O + light energy = C6H12O6 + 6O2 • J) 6CO2 + 6H2O + light energy = CH12O6 + 6CO2 • K) C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. • L) 6CO2 + 6H2O + no energy = C6H12O6 + 6O2 • M) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • N.) C6H12O6 + 6O2 = light energy + 6CO2 + 6H2O. Copyright © 2010 Ryan P. Murphy
- 179. • Which one is the equation for photosynthesis, and which one is the equation for respiration? • A) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • B) C6H12O6 + O2 = Released energy + 6CO2 + 6H2O. • C) 6CO2 + 6O2 + light energy = C6H12O6 + 6H2O • D) 6CO2 + 12H2O + light energy = C6H12O6 + 6H2O • E) C6H12O6 + 6O2 = Released energy + 6O2 + H2O. • F) 6CO2 + 6H2O + light energy = C6H12O6 + 6O2 • G) 6CO2 + 6H2O + light energy = C6H12O6 + 6CO2 • H) C6H12O6 + 6CO2 = Released energy + 6CO2 + 6H2O. • I) 6CO2 + 6H6O + light energy = C6H12O6 + 6O2 • J) 6CO2 + 6H2O + light energy = CH12O6 + 6CO2 • K) C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. • L) 6CO2 + 6H2O + no energy = C6H12O6 + 6O2 • M) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • N.) C6H12O6 + 6O2 = light energy + 6CO2 + 6H2O. Copyright © 2010 Ryan P. Murphy
- 180. • Which one is the equation for photosynthesis, and which one is the equation for respiration? • A) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • B) C6H12O6 + O2 = Released energy + 6CO2 + 6H2O. • C) 6CO2 + 6O2 + light energy = C6H12O6 + 6H2O • D) 6CO2 + 12H2O + light energy = C6H12O6 + 6H2O • E) C6H12O6 + 6O2 = Released energy + 6O2 + H2O. • F) 6CO2 + 6H2O + light energy = C6H12O6 + 6O2 • G) 6CO2 + 6H2O + light energy = C6H12O6 + 6CO2 • H) C6H12O6 + 6CO2 = Released energy + 6CO2 + 6H2O. • I) 6CO2 + 6H6O + light energy = C6H12O6 + 6O2 • J) 6CO2 + 6H2O + light energy = CH12O6 + 6CO2 • K) C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. • L) 6CO2 + 6H2O + no energy = C6H12O6 + 6O2 • M) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • N.) C6H12O6 + 6O2 = light energy + 6CO2 + 6H2O. Copyright © 2010 Ryan P. Murphy
- 181. • Which one is the equation for photosynthesis, and which one is the equation for respiration? • A) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • B) C6H12O6 + O2 = Released energy + 6CO2 + 6H2O. • C) 6CO2 + 6O2 + light energy = C6H12O6 + 6H2O • D) 6CO2 + 12H2O + light energy = C6H12O6 + 6H2O • E) C6H12O6 + 6O2 = Released energy + 6O2 + H2O. • F) 6CO2 + 6H2O + light energy = C6H12O6 + 6O2 • G) 6CO2 + 6H2O + light energy = C6H12O6 + 6CO2 • H) C6H12O6 + 6CO2 = Released energy + 6CO2 + 6H2O. • I) 6CO2 + 6H6O + light energy = C6H12O6 + 6O2 • J) 6CO2 + 6H2O + light energy = CH12O6 + 6CO2 • K) C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. • L) 6CO2 + 6H2O + no energy = C6H12O6 + 6O2 • M) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • N.) C6H12O6 + 6O2 = light energy + 6CO2 + 6H2O. Copyright © 2010 Ryan P. Murphy
- 182. • Which one is the equation for photosynthesis, and which one is the equation for respiration? • A) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • B) C6H12O6 + O2 = Released energy + 6CO2 + 6H2O. • C) 6CO2 + 6O2 + light energy = C6H12O6 + 6H2O • D) 6CO2 + 12H2O + light energy = C6H12O6 + 6H2O • E) C6H12O6 + 6O2 = Released energy + 6O2 + H2O. • F) 6CO2 + 6H2O + light energy = C6H12O6 + 6O2 • G) 6CO2 + 6H2O + light energy = C6H12O6 + 6CO2 • H) C6H12O6 + 6CO2 = Released energy + 6CO2 + 6H2O. • I) 6CO2 + 6H6O + light energy = C6H12O6 + 6O2 • J) 6CO2 + 6H2O + light energy = CH12O6 + 6CO2 • K) C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. • L) 6CO2 + 6H2O + no energy = C6H12O6 + 6O2 • M) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • N.) C6H12O6 + 6O2 = light energy + 6CO2 + 6H2O. Copyright © 2010 Ryan P. Murphy Law Conservation of Matter:
- 183. • Which one is the equation for photosynthesis, and which one is the equation for respiration? • A) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • B) C6H12O6 + O2 = Released energy + 6CO2 + 6H2O. • C) 6CO2 + 6O2 + light energy = C6H12O6 + 6H2O • D) 6CO2 + 12H2O + light energy = C6H12O6 + 6H2O • E) C6H12O6 + 6O2 = Released energy + 6O2 + H2O. • F) 6CO2 + 6H2O + light energy = C6H12O6 + 6O2 • G) 6CO2 + 6H2O + light energy = C6H12O6 + 6CO2 • H) C6H12O6 + 6CO2 = Released energy + 6CO2 + 6H2O. • I) 6CO2 + 6H6O + light energy = C6H12O6 + 6O2 • J) 6CO2 + 6H2O + light energy = CH12O6 + 6CO2 • K) C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. • L) 6CO2 + 6H2O + no energy = C6H12O6 + 6O2 • M) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • N.) C6H12O6 + 6O2 = light energy + 6CO2 + 6H2O. Copyright © 2010 Ryan P. Murphy Law Conservation of Matter: In any physical or chemical reaction, matter cannot be created or destroyed.
- 184. • Which one is the equation for photosynthesis, and which one is the equation for respiration? • A) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • B) C6H12O6 + O2 = Released energy + 6CO2 + 6H2O. • C) 6CO2 + 6O2 + light energy = C6H12O6 + 6H2O • D) 6CO2 + 12H2O + light energy = C6H12O6 + 6H2O • E) C6H12O6 + 6O2 = Released energy + 6O2 + H2O. • F) 6CO2 + 6H2O + light energy = C6H12O6 + 6O2 • G) 6CO2 + 6H2O + light energy = C6H12O6 + 6CO2 • H) C6H12O6 + 6CO2 = Released energy + 6CO2 + 6H2O. • I) 6CO2 + 6H6O + light energy = C6H12O6 + 6O2 • J) 6CO2 + 6H2O + light energy = CH12O6 + 6CO2 • K) C6H12O6 + 6O2 = Released energy + 6CO2 + 6H2O. • L) 6CO2 + 6H2O + no energy = C6H12O6 + 6O2 • M) 6O2 + 6H2O + light energy = C12H6O6 + 6O2 • N.) C6H12O6 + 6O2 = light energy + 6CO2 + 6H2O. Copyright © 2010 Ryan P. Murphy Law Conservation of Matter: In any physical or chemical reaction, matter cannot be created or destroyed. Just changes form.
- 187. Mitochondria
- 188. Mitochondria
- 198. • Try Again __CH4 + __O2 --> __CO2 + __H2O___ NaCl + __ BeF2 --> __ NaF +__ BeCl2 Na Cl Be F
- 199. • Try Again BOXES!!! __CH4 + __O2 --> __CO2 + __H2O___ NaCl + __ BeF2 --> __ NaF +__ BeCl2 Na Cl Be F
- 200. • Try Again BOXES!!! __CH4 + __O2 --> __CO2 + __H2O___ NaCl + __ BeF2 --> __ NaF +__ BeCl2 Na Cl Be F
- 201. __CH4 + __O2 --> __CO2 + __H2O___ NaCl + __ BeF2 --> __ NaF +__ BeCl2 Na Cl Be F
- 202. __CH4 + __O2 --> __CO2 + __H2O___ NaCl + __ BeF2 --> __ NaF +__ BeCl2 Na Cl Be F
- 203. __CH4 + __O2 --> __CO2 + __H2O___ NaCl + __ BeF2 --> __ NaF +__ BeCl2 Na Cl Be F 1 1
- 204. __CH4 + __O2 --> __CO2 + __H2O___ NaCl + __ BeF2 --> __ NaF +__ BeCl2 Na Cl Be F 1 1
- 205. __CH4 + __O2 --> __CO2 + __H2O___ NaCl + __ BeF2 --> __ NaF +__ BeCl2 Na Cl Be F 1 1 1 2
- 206. __CH4 + __O2 --> __CO2 + __H2O___ NaCl + __ BeF2 --> __ NaF +__ BeCl2 Na Cl Be F 1 1 1 2
- 207. __CH4 + __O2 --> __CO2 + __H2O___ NaCl + __ BeF2 --> __ NaF +__ BeCl2 Na Cl Be F 1 1 1 2 1 1
- 208. __CH4 + __O2 --> __CO2 + __H2O___ NaCl + __ BeF2 --> __ NaF +__ BeCl2 Na Cl Be F 1 1 1 2 1 1
- 209. __CH4 + __O2 --> __CO2 + __H2O___ NaCl + __ BeF2 --> __ NaF +__ BeCl2 Na Cl Be F 1 1 1 2 1 1 2 1
- 210. __CH4 + __O2 --> __CO2 + __H2O___ NaCl + __ BeF2 --> __ NaF +__ BeCl2 Na Cl Be F 1 1 1 2 1 1 2 1
- 211. __CH4 + __O2 --> __CO2 + __H2O___ NaCl + __ BeF2 --> __ NaF +__ BeCl2 Na Cl Be F 1 1 1 2 1 1 2 1
- 212. __CH4 + __O2 --> __CO2 + __H2O___ NaCl + __ BeF2 --> __ NaF +__ BeCl2 Na Cl Be F
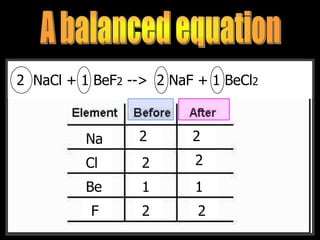
- 213. __CH4 + __O2 --> __CO2 + __H2O2 NaCl + 1 BeF2 --> 2 NaF + 1 BeCl2 Na Cl Be F 2 2 2 2 1 1 2 2
- 215. __CH4 + __O2 --> __CO2 + __H2O__Mg + __Mn2O3 --> __MgO + __Mn Mg Mn O F
- 216. __CH4 + __O2 --> __CO2 + __H2O__Mg + __Mn2O3 --> __MgO + __Mn Mg Mn O F
- 217. __CH4 + __O2 --> __CO2 + __H2O__Mg + __Mn2O3 --> __MgO + __Mn Mg Mn O F
- 218. __CH4 + __O2 --> __CO2 + __H2O__Mg + __Mn2O3 --> __MgO + __Mn Mg Mn O F
- 219. __CH4 + __O2 --> __CO2 + __H2O__Mg + __Mn2O3 --> __MgO + __Mn Mg Mn O F 1 1
- 220. __CH4 + __O2 --> __CO2 + __H2O__Mg + __Mn2O3 --> __MgO + __Mn Mg Mn O F 1 1
- 221. __CH4 + __O2 --> __CO2 + __H2O__Mg + __Mn2O3 --> __MgO + __Mn Mg Mn O F 1 1 2 1
- 222. __CH4 + __O2 --> __CO2 + __H2O__Mg + __Mn2O3 --> __MgO + __Mn Mg Mn O F 1 1 2 1
- 223. __CH4 + __O2 --> __CO2 + __H2O__Mg + __Mn2O3 --> __MgO + __Mn Mg Mn O F 1 1 2 1 3 1
- 224. __CH4 + __O2 --> __CO2 + __H2O__Mg + __Mn2O3 --> __MgO + __Mn Mg Mn O F 1 1 2 1 3 1
- 225. __CH4 + __O2 --> __CO2 + __H2O__Mg + __Mn2O3 --> __MgO + __Mn Mg Mn O F 1 1 2 1 3 1
- 226. • Answer: See if this right? __CH4 + __O2 --> __CO2 + __H2O3 Mg + 1 Mn2O3 --> 3 MgO + 3 Mn Mg Mn O F 1 1 2 1 3 1
- 227. • Answer: See if this right? __CH4 + __O2 --> __CO2 + __H2O3 Mg + 1 Mn2O3 --> 3 MgO + 3 Mn Mg Mn O F 1 1 2 1 3 1
- 228. __CH4 + __O2 --> __CO2 + __H2O3 Mg + 1 Mn2O3 --> 3 MgO + 3 Mn Mg Mn O F 1 1 2 1 3 1 3 3
- 229. __CH4 + __O2 --> __CO2 + __H2O3 Mg + 1 Mn2O3 --> 3 MgO + 3 Mn Mg Mn O F 1 1 2 1 3 1 3 3
- 230. __CH4 + __O2 --> __CO2 + __H2O3 Mg + 1 Mn2O3 --> 3 MgO + 3 Mn Mg Mn O F 1 1 2 1 3 1 3 3 2 3
- 231. __CH4 + __O2 --> __CO2 + __H2O3 Mg + 1 Mn2O3 --> 3 MgO + 3 Mn Mg Mn O F 1 1 2 1 3 1 3 3 2 3
- 232. __CH4 + __O2 --> __CO2 + __H2O3 Mg + 1 Mn2O3 --> 3 MgO + 3 Mn Mg Mn O F 1 1 2 1 3 1 3 3 2 3
- 233. __CH4 + __O2 --> __CO2 + __H2O3 Mg + 1 Mn2O3 --> 3 MgO + 3 Mn Mg Mn O F 1 1 2 1 3 1 3 3 2 3
- 234. __CH4 + __O2 --> __CO2 + __H2O3 Mg + 1 Mn2O3 --> 3 MgO + 3 Mn Mg Mn O F 1 1 2 1 3 1 3 3 2 3
- 235. __CH4 + __O2 --> __CO2 + __H2O3 Mg + 1 Mn2O3 --> 3 MgO + 3 Mn Mg Mn O F 1 1 2 1 3 1 3 3 2 3
- 236. __CH4 + __O2 --> __CO2 + __H2O3 Mg + 1 Mn2O3 --> 3 MgO + 1 Mn Mg Mn O F 1 1 2 1 3 1
- 237. __CH4 + __O2 --> __CO2 + __H2O3 Mg + 1 Mn2O3 --> 3 MgO + 1 Mn Mg Mn O F 1 1 2 1 3 1 3 3 2 1
- 238. __CH4 + __O2 --> __CO2 + __H2O3 Mg + 1 Mn2O3 --> 3 MgO + 1 Mn Mg Mn O F 1 1 2 1 3 1 3 3 2 1
- 239. __CH4 + __O2 --> __CO2 + __H2O3 Mg + 1 Mn2O3 --> 3 MgO + 1 Mn Mg Mn O F 1 1 2 1 3 1 3 3 2 1
- 240. • Answer: See if this right? __CH4 + __O2 --> __CO2 + __H2O3 Mg + 1 Mn2O3 --> 3 MgO + 2 Mn Mg Mn O F 1 1 2 1 3 1
- 241. • Answer: See if this right? • This is a balanced equation. __CH4 + __O2 --> __CO2 + __H2O3 Mg + 1 Mn2O3 --> 3 MgO + 2 Mn Mg Mn O F 1 1 2 1 3 1 3 3 2 2 3 3
- 242. • An easy one… • ___ Na + ___Cl ____ NaCl • The original equation is Na + Cl = NaCl. • The thing is, chlorine is one of 7 elements that doesn't like to be alone, so it's always 'Cl2', making the equation Na + Cl2 = NaCl. • However, this is no longer balanced. So what you do is add a '2' onto NaCl, making it Na + Cl2 = 2NaCl. • Now the chlorine is balanced, but the sodium isn't. • After that, to balance the sodium, you add a '2' in front of 'Na' making the equation 2Na + Cl2 = 2NaCl. + -
- 243. • An easy one… • ___ Na + ___Cl ____ NaCl • The original equation is Na + Cl = NaCl. • The thing is, chlorine is one of 7 elements that doesn't like to be alone, so it's always 'Cl2', making the equation Na + Cl2 = NaCl. • However, this is no longer balanced. So what you do is add a '2' onto NaCl, making it Na + Cl2 = 2NaCl. • Now the chlorine is balanced, but the sodium isn't. • After that, to balance the sodium, you add a '2' in front of 'Na' making the equation 2Na + Cl2 = 2NaCl. + -
- 244. • An easy one… • ___ Na + ___Cl ____ NaCl • The original equation is Na + Cl = NaCl. • The thing is, chlorine is one of 7 elements that doesn't like to be alone, so it's always 'Cl2', making the equation Na + Cl2 = NaCl. • However, this is no longer balanced. So what you do is add a '2' onto NaCl, making it Na + Cl2 = 2NaCl. • Now the chlorine is balanced, but the sodium isn't. • After that, to balance the sodium, you add a '2' in front of 'Na' making the equation 2Na + Cl2 = 2NaCl. + -
- 245. • An easy one… • ___ Na + ___Cl ____ NaCl • The original equation is Na + Cl = NaCl. • The thing is, chlorine is one of 7 elements that doesn't like to be alone, so it's always 'Cl2', making the equation Na + Cl2 = NaCl. • However, this is no longer balanced. So what you do is add a '2' onto NaCl, making it Na + Cl2 = 2NaCl. • Now the chlorine is balanced, but the sodium isn't. • After that, to balance the sodium, you add a '2' in front of 'Na' making the equation 2Na + Cl2 = 2NaCl. + 2 -
- 246. • An easy one… • ___ Na + ___Cl ____ NaCl • The original equation is Na + Cl = NaCl. • The thing is, chlorine is one of 7 elements that doesn't like to be alone, so it's always 'Cl2', making the equation Na + Cl2 = NaCl. • However, this is no longer balanced. So what you do is add a '2' onto 2 Na, making it 2 Na + Cl2 = 2NaCl. • Now the chlorine is balanced, but the sodium isn't. • After that, to balance the sodium, you add a '2' in front of 'Na' making the equation 2Na + Cl2 = 2NaCl. + 2 -
- 247. • An easy one… • ___ Na + ___Cl ____ NaCl • The original equation is Na + Cl = NaCl. • The thing is, chlorine is one of 7 elements that doesn't like to be alone, so it's always 'Cl2', making the equation Na + Cl2 = NaCl. • However, this is no longer balanced. So what you do is add a '2' onto 2 Na, making it 2 Na + Cl2 = 2NaCl. • Now the chlorine is balanced, but the sodium isn't. • After that, to balance the sodium, you add a '2' in front of 'Na' making the equation 2Na + Cl2 = 2NaCl. + 2 -
- 248. • An easy one… • ___ Na + ___Cl ____ NaCl • The original equation is Na + Cl = NaCl. • The thing is, chlorine is one of 7 elements that doesn't like to be alone, so it's always 'Cl2', making the equation Na + Cl2 = NaCl. • However, this is no longer balanced. So what you do is add a '2' onto 2 Na, making it 2 Na + Cl2 = 2NaCl. • Now the chlorine is balanced, but the sodium isn't. • After that, to balance the sodium, you add a '2' in front of 'Na' making the equation 2Na + Cl2 = 2NaCl. + 2 -
- 249. • An easy one… • ___ Na + ___Cl ____ NaCl • The original equation is Na + Cl = NaCl. • The thing is, chlorine is one of 7 elements that doesn't like to be alone, so it's always 'Cl2', making the equation Na + Cl2 = NaCl. • However, this is no longer balanced. So what you do is add a '2' onto 2 Na, making it 2 Na + Cl2 = 2NaCl. • Now the chlorine is balanced, but the sodium isn't. • After that, to balance the sodium, you add a '2' in front of 'Na' making the equation 2Na + Cl2 = 2NaCl. + 2 -
- 250. • An easy one… • ___ Na + ___Cl ____ NaCl • The original equation is Na + Cl = NaCl. • The thing is, chlorine is one of 7 elements that doesn't like to be alone, so it's always 'Cl2', making the equation Na + Cl2 = NaCl. • However, this is no longer balanced. So what you do is add a '2' onto 2 Na, making it 2 Na + Cl2 = 2NaCl. • Now the chlorine is balanced, but the sodium isn't. • After that, to balance the sodium, you add a '2' in front of 'Na' making the equation 2Na + Cl2 = 2NaCl. + 2 -
- 251. • An easy one… • ___ Na + ___Cl ____ NaCl • The original equation is Na + Cl = NaCl. • The thing is, chlorine is one of 7 elements that doesn't like to be alone, so it's always 'Cl2', making the equation Na + Cl2 = NaCl. • However, this is no longer balanced. So what you do is add a '2' onto 2 Na, making it 2 Na + Cl2 = 2NaCl. • Now the chlorine is balanced, but the sodium isn't. • After that, to balance the sodium, you add a '2' in front of 'Na' making the equation 2Na + Cl2 = 2NaCl. + 2 -
- 252. • Complete the questions for homework.
- 253. __CH4 + __O2 --> __CO2 + __H2O3 Mg + 1 Mn2O3 --> 3 MgO + 2 Mn Mg Mn O F 1 1 2 1 3 1 3 3 2 2 3 3 Note – There are other methods to balance equations without the use of tables and inventory charts. The following links can show you other methods. http://www.sky- web.net/science/balancing_chemical_equations.htm http://chemistry.about.com/cs/stoichiometry/a/aa042903a.h tm http://www.fordhamprep.org/gcurran/sho/sho/lessons/lesso n81.htm
- 254. • Okay, we now can understand it. Let’s learn how to shorten this process a bit. – http://www.youtube.com/watch?v=vO9VgitCx04
- 255. • Another way to balance chemical Equations. • Video Link! Khan Academy - Balancing Chemical Equations. – http://www.youtube.com/watch?v=RnGu3xO2h74
- 256. • Online Quiz Link! Balancing Chemical Equations. • http://education.jlab.org/elementbalancing/ index.html
- 257. • Video Link! Balancing Chemical Equations. – http://www.youtube.com/watch?v=_B735turDo M&feature=em-subs_digest-vrecs
- 258. • Video Link! Khan Academy, Balancing Chemical Equations. • http://www.khanacademy.org/video/balanc ing-chemical- equations?playlist=Chemistry
- 259. • Activity Simulator: Balancing Chemical Equations. – http://phet.colorado.edu/en/simulation/balanci ng-chemical-equations
- 260. • Blank inventory charts available for worksheets on next slide.
- 261. • Blank inventory charts available for worksheets on next slide. Many more equations / worksheets can be found at… http://chemistry.about.com/library/formulabalance.pdf Answers http://chemistry.about.com/library/formulabalance2.pdf Answers http://chemistry.about.com/library/formulabalance3.pdf Answers
- 262. • Activity Sheet! Balancing Unbalanced Chemical Equations. (New Problems) –Do your best as this can be very difficult for some. –Use the Inventory Box Method or the one learned from other sources.
- 263. • Endothermic
- 264. • Endothermic – Endo = Inside
- 265. • Endothermic and Exothermic Reactions – Endo = Inside
- 266. • Endothermic and Exothermic Reactions – Endo = Inside
- 267. • Endothermic and Exothermic Reactions – Endo = Inside – Exo = Outside
- 268. • Law Conservation of Energy – Everything is trying to get to the same temperature. • Heat goes from Hot to Cold
- 269. • Law Conservation of Energy – Everything is trying to get to the same temperature. • Heat goes from Hot to Cold
- 270. • Energy cannot be created or destroyed but can diminish in quality from useful to less useful. Copyright © 2010 Ryan P. Murphy
- 271. • Energy comes from somewhere – Nothing is free. Copyright © 2010 Ryan P. Murphy
- 272. • Energy can be transformed from one form to another. Copyright © 2010 Ryan P. Murphy
- 273. • Energy can be transformed from one form to another. Copyright © 2010 Ryan P. Murphy
- 274. • Energy can be transformed from one form to another. Copyright © 2010 Ryan P. Murphy
- 275. • Energy can be transformed from one form to another. Copyright © 2010 Ryan P. Murphy
- 276. • Energy can be transformed from one form to another. Copyright © 2010 Ryan P. Murphy
- 277. • Energy can be transformed from one form to another. Copyright © 2010 Ryan P. Murphy
- 278. • Energy can be transformed from one form to another. Copyright © 2010 Ryan P. Murphy
- 279. • Energy can be transformed from one form to another. Copyright © 2010 Ryan P. Murphy
- 280. • To change from one state to another (from solid, to liquid, to gas, etc.) takes energy.
- 281. • To change from one state to another (from solid, to liquid, to gas, etc.) takes energy. – To change from one molecular structure to another requires energy.
- 282. • Endo and Exothermic Reactions Available Sheet.
- 283. Exothermic Reactions: Chemical reactions that releases energy in the form of heat, light, or sound. The products contain less energy than the reactants Heat is lost to the surroundings.
- 284. Exothermic Reactions: Chemical reactions that releases energy in the form of heat, light, or sound. The products contain less energy than the reactants Heat is lost to the surroundings.
- 285. Exothermic Reactions: Chemical reactions that releases energy in the form of heat, light, or sound. The products contain less energy than the reactants Heat is lost to the surroundings.
- 286. Exothermic Reactions: Chemical reactions that releases energy in the form of heat, light, or sound. The products contain less energy than the reactants Heat is lost to the surroundings.
- 287. • Video Link! Gummy Bear meets Potassium Chlorate. – http://www.youtube.com/watch?v=bXScgXleLX8
- 288. • Potential Energy – When two atoms form a strong covalent or ionic bond, chemical energy is converted into other forms of energy, usually in the form of heat and light.
- 289. • Potential Energy – When two atoms form a strong covalent or ionic bond, chemical energy is converted into other forms of energy, usually in the form of heat and light.
- 290. • Potential Energy – When two atoms form a strong covalent or ionic bond, chemical energy is converted into other forms of energy, usually in the form of heat and light.
- 291. • Potential Energy – When two atoms form a strong covalent or ionic bond, chemical energy is converted into other forms of energy, usually in the form of heat and light. The stronger the bond the more energy is released.
- 292. • Potential Energy – When two atoms form a strong covalent or ionic bond, chemical energy is converted into other forms of energy, usually in the form of heat and light. The stronger the bond the more energy is released. Strong bonds do require more energy to break.
- 293. • To figure out if a reaction is exothermic or endothermic.
- 294. • To figure out if a reaction is exothermic or endothermic. – Observe how the temperature of the surroundings changes. – An exothermic process releases heat that causes the temperature of the immediate surroundings to increase. – An endothermic process absorbs heat and makes the surroundings colder.
- 295. • To figure out if a reaction is exothermic or endothermic. – Observe how the temperature of the surroundings changes. – An exothermic process releases heat that causes the temperature of the immediate surroundings to increase. – An endothermic process absorbs heat and makes the surroundings colder.
- 296. • To figure out if a reaction is exothermic or endothermic. – Observe how the temperature of the surroundings changes. – An exothermic process releases heat that causes the temperature of the immediate surroundings to increase. – An endothermic process absorbs heat and makes the surroundings colder.
- 297. • To figure out if a reaction is exothermic or endothermic. – Observe how the temperature of the surroundings changes. – An exothermic process releases heat that causes the temperature of the immediate surroundings to increase. – An endothermic process absorbs heat and makes the surroundings colder.
- 298. • To figure out if a reaction is exothermic or endothermic. – Observe how the temperature of the surroundings changes. – An exothermic process releases heat that causes the temperature of the immediate surroundings to increase. – An endothermic process absorbs heat and makes the surroundings colder.
- 299. • To figure out if a reaction is exothermic or endothermic. – Observe how the temperature of the surroundings changes. – An exothermic process releases heat that causes the temperature of the immediate surroundings to increase. – An endothermic process absorbs heat and makes the surroundings colder.
- 300. • To figure out if a reaction is exothermic or endothermic. – Observe how the temperature of the surroundings changes. – An exothermic process releases heat that causes the temperature of the immediate surroundings to increase. – An endothermic process absorbs heat and makes the surroundings colder.
- 301. • To figure out if a reaction is exothermic or endothermic. – Observe how the temperature of the surroundings changes. – An exothermic process releases heat that causes the temperature of the immediate surroundings to increase. – An endothermic process absorbs heat and makes the surroundings colder.
- 302. • Which is endothermic and which is exothermic?
- 303. • Which is endothermic and which is exothermic?
- 304. • Which is endothermic and which is exothermic?
- 305. • Which is endothermic and which is exothermic?
- 306. • Which is endothermic and which is exothermic?
- 307. • Which is endothermic and which is exothermic?
- 308. • Which is endothermic and which is exothermic?
- 309. • Which is endothermic and which is exothermic?
- 310. • Which is endothermic and which is exothermic?
- 311. • Which is endothermic and which is exothermic?
- 312. • Which is endothermic and which is exothermic?
- 313. • Which is endothermic and which is exothermic?
- 319. Energy always constant Reactants Products
- 320. Energy always constant Reactants Products
- 321. Energy always constant Reactants Products Heat and Light
- 322. Energy always constant Reactants Products Heat and Light Heat and Light
- 323. Energy always constant Reactants Products Heat and Light Heat and Light Chemical Energy
- 324. Energy always constant Reactants Products Heat and Light Heat and Light Chemical Energy
- 325. Energy always constant Reactants Products Heat and Light Heat and Light Chemical Energy
- 326. Energy always constant Reactants Products Heat and Light Heat and Light Chemical Energy Chemical Energy is converted to heat and Light.
- 327. Energy always constant Reactants Products Heat and Light Heat and Light Chemical Energy Chemical Energy is converted to heat and Light. The more chemical energy the more heat and light.
- 328. Energy always constant Reactants Products Heat and Light Heat and Light Chemical Energy Chemical Energy is converted to heat and Light. The more chemical energy the more heat and light. (Constant)
- 329. • Activity! Whoosh Bottle – Search Whoosh Bottle to learn more. – http://www.youtube.com/watch?v=AS8TDpFP0 OQ
- 331. • Activity! Making Elephant Toothpaste. – Safety goggles and gloves are needed.
- 332. • Activity! Making Elephant Toothpaste. – Safety goggles and gloves are needed. Demonstration at… http://chemistry.about.com/od/chemistrydemonstrations/a/ elephanttooth.htm
- 333. • Endo and Exothermic Reactions Avaialble Sheet.
- 334. • Materials – Empty 20 oz clear soda bottle. – Hydrogen peroxide (3% from store or 8% from cosmetic store) – Active yeast – Warm water – Liquid dish soap – Food coloring – Spill tray.
- 335. • Procedure: – Mix 120 ml of hydrogen peroxide with 60 ml of liquid dish soap and a few drops of food coloring. Add this mixture to the empty soda bottle and place it on the spill tray. – In a separate container, mix one packet (1 teaspoon or 11 ml / 7 grams) of active yeast with a little warm water (2 tablespoons / 30 ml) and let it sit for 5 minutes. – Remove clumps of yeast so you just add the liquid. – Pour the yeast mixture into the soda bottle with a funnel and watch the reaction. – Feel the container for heat and look for steam (Exothermic) – All contents can be disposed of in the sink.
- 337. • Elephant Toothpaste – The chemical formula for hydrogen peroxide is H2O2. – Hydrogen peroxide is not stable so it is always decomposing into water and oxygen. – This occurs slowly under normal conditions. – Yeast make the reaction go much faster and the dishwashing soap creates the foam. – The overall equation for this reaction is: – 2 H2O2(aq) --> 2 H2O(l) + O2(g) HEAT
- 338. • Elephant Toothpaste – The chemical formula for hydrogen peroxide is H2O2. – Hydrogen peroxide is not stable so it is always decomposing into water and oxygen. – This occurs slowly under normal conditions. – Yeast make the reaction go much faster and the dishwashing soap creates the foam. – The overall equation for this reaction is: – 2 H2O2(aq) --> 2 H2O(l) + O2(g) HEAT
- 339. • Elephant Toothpaste – The chemical formula for hydrogen peroxide is H2O2. – Hydrogen peroxide is not stable so it’s always decomposing into water and oxygen. – This occurs slowly under normal conditions. – Yeast make the reaction go much faster and the dishwashing soap creates the foam. – The overall equation for this reaction is: – 2 H2O2(aq) --> 2 H2O(l) + O2(g) HEAT
- 340. • Elephant Toothpaste – The chemical formula for hydrogen peroxide is H2O2. – Hydrogen peroxide is not stable so it’s always decomposing into water and oxygen. – This occurs slowly under normal conditions. – Yeast make the reaction go much faster and the dishwashing soap creates the foam. – The overall equation for this reaction is: – 2 H2O2(aq) --> 2 H2O(l) + O2(g) HEAT
- 341. • Elephant Toothpaste – The chemical formula for hydrogen peroxide is H2O2. – Hydrogen peroxide is not stable so it’s always decomposing into water and oxygen. – This occurs slowly under normal conditions. – Yeast make the reaction go much faster and the dishwashing soap creates the foam. – The overall equation for this reaction is: – 2 H2O2(aq) --> 2 H2O(l) + O2(g) HEAT
- 342. • Elephant Toothpaste – The chemical formula for hydrogen peroxide is H2O2. – Hydrogen peroxide is not stable so it’s always decomposing into water and oxygen. – This occurs slowly under normal conditions. – Yeast make the reaction go much faster and the dishwashing soap creates the foam. – The overall equation for this reaction is: – 2 H2O2(aq) --> 2 H2O(l) + O2(g) HEAT
- 343. • Elephant Toothpaste – The chemical formula for hydrogen peroxide is H2O2. – Hydrogen peroxide is not stable so it’s always decomposing into water and oxygen. – This occurs slowly under normal conditions. – Yeast make the reaction go much faster and the dishwashing soap creates the foam. – The overall equation for this reaction is: – H2O2(aq) + OI-(aq) → I-(aq) + H2O(l) + O2(g)
- 344. • Video Link! Exothermic Reaction “Elephant Toothpaste” – http://www.youtube.com/watch?v=4N0m95PExHY
- 345. • Endo and Exothermic Reactions Avaialble Sheet.
- 346. Endothermic reactions: These reactions absorb energy in order to proceed.
- 347. Endothermic reactions: These reactions absorb energy in order to proceed. The products contain more energy than the reactants, heat is taken in or absorbed from the surroundings.
- 348. Endothermic reactions: These reactions absorb energy in order to proceed. The products contain more energy than the reactants, heat is taken in or absorbed from the surroundings. A temperature drop is measured during the reaction.
- 349. Endothermic reactions: These reactions absorb energy in order to proceed. The products contain more energy than the reactants, heat is taken in or absorbed from the surroundings. A temperature drop is measured during the reaction.
- 350. Endothermic reactions: These reactions absorb energy in order to proceed. The products contain more energy than the reactants, heat is taken in or absorbed from the surroundings. A temperature drop is measured during the reaction.
- 351. • Activity! Endothermic Reaction – When baking soda mixes with vinegar it is an endothermic reaction. The vinegar and baking soda are changing from their individual molecular structures to a new molecular structure (Chemical Change). This molecular change requires energy (heat) which it absorbs from the surroundings. – NaHCO3 (aq) + CH3COOH (aq) ----> CO2 (g) + H2O (l) + CH3COONa (aq)
- 352. • Activity! Endothermic Reaction – When baking soda mixes with vinegar it’s an endothermic reaction. The vinegar and baking soda are changing from their individual molecular structures to a new molecular structure (Chemical Change). This molecular change requires energy (heat) which it absorbs from the surroundings. – NaHCO3 (aq) + CH3COOH (aq) ----> CO2 (g) + H2O (l) + CH3COONa (aq)
- 353. • Activity! Endothermic Reaction – When baking soda mixes with vinegar it’s an endothermic reaction. The vinegar and baking soda are changing from their individual molecular structures to a new molecular structure (Chemical Change). This molecular change requires energy (heat) which it absorbs from the surroundings. – NaHCO3 (aq) + CH3COOH (aq) ----> CO2 (g) + H2O (l) + CH3COONa (aq)
- 354. • Activity! Endothermic Reaction – When baking soda mixes with vinegar it’s an endothermic reaction. The vinegar and baking soda are changing from their individual molecular structures to a new molecular structure (Chemical Change). This molecular change requires energy (heat) which it absorbs from the surroundings. – NaHCO3 (aq) + CH3COOH (aq) ----> CO2 (g) + H2O (l) + CH3COONa (aq)
- 355. • Activity! Endothermic Reaction – When baking soda mixes with vinegar it’s an endothermic reaction. The vinegar and baking soda are changing from their individual molecular structures to a new molecular structure (Chemical Change). This molecular change requires energy (heat) which it absorbs from the surroundings. – NaHCO3 (aq) + CH3COOH (aq)
- 356. • Activity! Endothermic Reaction – When baking soda mixes with vinegar it’s an endothermic reaction. The vinegar and baking soda are changing from their individual molecular structures to a new molecular structure (Chemical Change). This molecular change requires energy (heat) which it absorbs from the surroundings. – NaHCO3 (aq) + CH3COOH (aq) ---->
- 357. • Activity! Endothermic Reaction – When baking soda mixes with vinegar it’s an endothermic reaction. The vinegar and baking soda are changing from their individual molecular structures to a new molecular structure (Chemical Change). This molecular change requires energy (heat) which it absorbs from the surroundings. – NaHCO3 (aq) + CH3COOH (aq) ----> CO2 (g) + H2O (l) + CH3COONa (aq)
- 358. • Activity! Endothermic or Exothermica Reaction – Alka-Seltzer in water Endo and exothermic reactions: Learn more at …. http://www.kentchemistry.com/links/Matter/En doExo.htm
- 359. • Endo and Exothermic Reactions Avaialble Sheet.
- 360. • Please create the following spreadsheet in your journal. Time Seconds (H2O) Temperature (Celsius) 30 60 90 120 Time Seconds (Alka) Temperature (Celsius) 30 60 90 120
- 361. • Procedure – Fill clear container with 100 ml of water. – Record temperature of water for 30, 60, 90, 120 seconds in spreadsheet. – Keep thermometer in container – Add 2 Alka-Seltzer tablets to the 100 ml of water. – Record temperature for 30, 60, 90,120 seconds on spreadsheet.
- 362. • Endo and Exothermic Reactions Avaiable Sheet.
- 363. • Please create a line graph of the temperature of the two in your journal. – Was the reaction Exothermic or Endothermic? 0 5 10 15 20 25 1 2 3 4 Alka-Seltzer Control 30 second intervals
- 364. • Please create a line graph of the temperature of the two in your journal. – Was the reaction Exothermic or Endothermic? 0 5 10 15 20 25 1 2 3 4 Alka-Seltzer Control 30 second intervals
- 365. • Please create a line graph of the temperature of the two in your journal. – Was the reaction Exothermic or Endothermic? 0 5 10 15 20 25 1 2 3 4 Alka-Seltzer Control 30 second intervals
- 366. • Endo and Exothermic Reactions Avaialble Sheet.
- 367. • Can you balance the equation for Alka-Seltzer and water. ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 368. • Can you balance the equation for Alka-Seltzer and water. ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 369. • Can you balance the equation for Alka-Seltzer and water. ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 370. • Can you balance the equation for Alka-Seltzer and water. ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 371. • Can you balance the equation for Alka-Seltzer and water. ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 372. • Can you balance the equation for Alka-Seltzer and water. ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 373. • Can you balance the equation for Alka-Seltzer and water. ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 374. • Can you balance the equation for Alka-Seltzer and water. ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 375. • Can you balance the equation for Alka-Seltzer and water. ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 376. • Can you balance the equation for Alka-Seltzer and water. ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 377. • Can you balance the equation for Alka-Seltzer and water. ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 378. • Can you balance the equation for Alka-Seltzer and water. ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 379. • Can you balance the equation for Alka-Seltzer and water. ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 380. • Can you balance the equation for Alka-Seltzer and water. ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 381. • Can you balance the equation for Alka-Seltzer and water. ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 382. • Can you balance the equation for Alka-Seltzer and water. ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 383. • Can you balance the equation for Alka-Seltzer and water. ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 384. • Can you balance the equation for Alka-Seltzer and water. ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 385. • Can you balance the equation for Alka-Seltzer and water. ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 386. • Can you balance the equation for Alka-Seltzer and water. ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 387. • Can you balance the equation for Alka-Seltzer and water. Will this balance? ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 388. • Can you balance the equation for Alka-Seltzer and water. Will this balance? ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 389. • Can you balance the equation for Alka-Seltzer and water. Will this balance? ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 390. • Can you balance the equation for Alka-Seltzer and water. Will this balance? ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 391. • Can you balance the equation for Alka-Seltzer and water. Will this balance? ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 392. • Can you balance the equation for Alka-Seltzer and water. Will this balance? ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 393. • Can you balance the equation for Alka-Seltzer and water. Will this balance? ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 394. • Can you balance the equation for Alka-Seltzer and water. Will this balance? ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 395. • Can you balance the equation for Alka-Seltzer and water. Will this balance? ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 396. • Can you balance the equation for Alka-Seltzer and water. Will this balance? ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 397. • Can you balance the equation for Alka-Seltzer and water. Will this balance? ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 398. • Can you balance the equation for Alka-Seltzer and water. Will this balance? ___ C6H8O7(aq) + ___NaHCO3(aq) → ___ H2O(l) + ___ CO2(g) + ___Na3C6H5O7(aq) citric acid + sodium bicarbonate → water + carbon dioxide + sodium citrate
- 399. • Which is endothermic and which is exothermic?
- 400. • Which is endothermic and which is exothermic?
- 401. • Which is endothermic and which is exothermic?
- 402. • Which is endothermic and which is exothermic?
- 403. • Which is endothermic and which is exothermic?
- 404. • Which is endothermic and which is exothermic?
- 405. • Oxidation and Reduction. – Optional Area of Focus
- 406. • Any reaction between an element or compound and oxygen is known as oxidation.
- 407. • Any reaction between an element or compound and oxygen is known as oxidation. – The reaction between magnesium metal and oxygen, for example, involves the oxidation of magnesium.
- 408. • Any reaction between an element or compound and oxygen is known as oxidation. – The reaction between magnesium metal and oxygen, for example, involves the oxidation of magnesium. • 2 Mg(s) + O2(g) 2 MgO(s)
- 409. • Any reaction between an element or compound and oxygen is known as oxidation. – The reaction between magnesium metal and oxygen, for example, involves the oxidation of magnesium. • 2 Mg(s) + O2(g) 2 MgO(s)
- 410. • Any reaction between an element or compound and oxygen is known as oxidation. – The reaction between magnesium metal and oxygen, for example, involves the oxidation of magnesium. • 2 Mg(s) + O2(g) 2 MgO(s)
- 411. • Any reaction between an element or compound and oxygen is known as oxidation. – The reaction between magnesium metal and oxygen, for example, involves the oxidation of magnesium. • 2 Mg(s) + O2(g) 2 MgO(s)
- 412. • Any reaction between an element or compound and oxygen is known as oxidation. – The reaction between magnesium metal and oxygen, for example, involves the oxidation of magnesium. • 2 Mg(s) + O2(g) 2 MgO(s)
- 413. • Any reaction between an element or compound and oxygen is known as oxidation. – The reaction between magnesium metal and oxygen, for example, involves the oxidation of magnesium. • 2 Mg(s) + O2(g) 2 MgO(s)
- 414. • Oxidation number of an element: The number of electrons lost, gained, or shared as a result of chemical bonding.
- 415. • Oxidation number of an element: The number of electrons lost, gained, or shared as a result of chemical bonding. – Oxidation: An increase in oxidation number
- 416. • Oxidation number of an element: The number of electrons lost, gained, or shared as a result of chemical bonding. – Oxidation: An increase in oxidation number
- 417. • Oxidation number of an element: The number of electrons lost, gained, or shared as a result of chemical bonding. – Oxidation: An increase in oxidation number – Reduction: An decrease in oxidation number
- 418. • Oxidation number of an element: The number of electrons lost, gained, or shared as a result of chemical bonding. – Oxidation: An increase in oxidation number – Reduction: An decrease in oxidation number
- 419. • Oxidation number of an element: The number of electrons lost, gained, or shared as a result of chemical bonding. – Oxidation: An increase in oxidation number – Reduction: An decrease in oxidation number
- 420. • Oxidation number of an element: The number of electrons lost, gained, or shared as a result of chemical bonding. – Oxidation: An increase in oxidation number – Reduction: An decrease in oxidation number
- 421. • What is this?
- 422. • What is this?
- 423. Oil Rig Oxidation Is Losing Electrons Reduction Is Gaining Electrons
- 424. Oil Rig Oxidation Is Losing Electrons Reduction Is Gaining Electrons
- 425. Oil Rig Oxidation Is Losing Electrons Reduction Is Gaining Electrons
- 426. Oil Rig Oxidation Is Losing Electrons Reduction Is Gaining Electrons
- 427. Oil Rig Oxidation Is Losing Electrons Reduction Is Gaining Electrons
- 428. Oil Rig Oxidation Is Losing Electrons Reduction Is Gaining Electrons
- 429. Oil Rig Oxidation Is Losing Electrons Reduction Is Gaining Electrons
- 430. Oil Rig Oxidation Is Losing Electrons Reduction Is Gaining Electrons “What, gaining electrons is reduction?” “That doesn’t make sense.”
- 431. • What’s this? LEO says GER • Losing • Electrons • Oxidation, • Gaining • Electrons • Reduction
- 432. • What’s this? LEO says GER • Losing • Electrons • Oxidation, • Gaining • Electrons • Reduction
- 433. • What’s this? LEO says GER • Losing • Electrons • Oxidation, • Gaining • Electrons • Reduction
- 434. • What’s this? LEO says GER • Losing • Electrons • Oxidation, • Gaining • Electrons • Reduction
- 435. • What’s this? LEO says GER • Losing • Electrons • Oxidation, • Gaining • Electrons • Reduction
- 436. • What’s this? LEO says GER • Losing • Electrons • Oxidation • Gaining • Electrons • Reduction
- 437. • What’s this? LEO says GER • Losing • Electrons • Oxidation • Gaining • Electrons • Reduction
- 441. To oxidize an atom or molecule means you have increased its overall positive charge.
- 442. To oxidize an atom or molecule means you have increased its overall positive charge. Removing electrons does this.
- 443. To oxidize an atom or molecule means you have increased its overall positive charge. Removing electrons does this. Atoms or molecules that give up electrons (or become oxidized) are electron donors.
- 444. To oxidize an atom or molecule means you have increased its overall positive charge. Removing electrons does this. Atoms or molecules that give up electrons (or become oxidized) are electron donors.
- 445. To oxidize an atom or molecule means you have increased its overall positive charge. Removing electrons does this. Atoms or molecules that give up electrons (or become oxidized) are electron donors.
- 446. To oxidize an atom or molecule means you have increased its overall positive charge. Removing electrons does this. Atoms or molecules that give up electrons (or become oxidized) are electron donors. Atoms or molecules that take on electrons (or become reduced) are called electron acceptors.
- 450. Atoms or molecules that take on electrons (or become reduced) are called electron acceptors.
- 454. To oxidize an atom or molecule means you have increased its overall positive charge. Removing electrons does this. Atoms or molecules that give up electrons (or become oxidized) are electron donors.
- 455. To oxidize an atom or molecule means you have increased its overall positive charge. Removing electrons does this. Atoms or molecules that give up electrons (or become oxidized) are electron donors.
- 456. To oxidize an atom or molecule means you have increased its overall positive charge. Removing electrons does this. Atoms or molecules that give up electrons (or become oxidized) are electron donors.
- 457. • Rust: The degree of oxidation of an atom in a chemical compound.
- 462. The Na starts out with an oxidation # of (0) and ends with and oxidation # of 1+.
- 463. The Na starts out with an oxidation # of (0) and ends with and oxidation # of 1+. It has been oxidized from a sodium atom to a positive sodium ion.
- 464. The Na starts out with an oxidation # of (0) and ends with and oxidation # of 1+. It has been oxidized from a sodium atom to a positive sodium ion. The Cl2 also starts with an oxidation # of (0), and ends with an oxidation number of 1-
- 465. The Na starts out with an oxidation # of (0) and ends with and oxidation # of 1+. It has been oxidized from a sodium atom to a positive sodium ion. The Cl2 also starts with an oxidation # of (0), and ends with an oxidation number of 1- It has been reduced from chlorine atoms to negative chloride ions.
- 466. • Fe (metal) + Cu2+ Fe2+ + Cu (metal) – Fe donates two electrons to the Cu2+ to form Cu (metal). The Fe lost 2 electrons, so it was oxidized. – The Cu2+ gained 2 electrons, so it was reduced.
- 467. • Fe (metal) + Cu2+ Fe2+ + Cu (metal) – Fe donates two electrons to the Cu2+ to form Cu (metal). The Fe lost 2 electrons, so it was oxidized. – The Cu2+ gained 2 electrons, so it was reduced.
- 468. • Fe (metal) + Cu2+ Fe2+ + Cu (metal) – Fe donates two electrons to the Cu2+ to form Cu (metal). The Fe lost 2 electrons, so it was oxidized. – The Cu2+ gained 2 electrons, so it was reduced.
- 469. • Fe (metal) + Cu2+ Fe2+ + Cu (metal) – Fe donates two electrons to the Cu2+ to form Cu (metal). The Fe lost 2 electrons, so it was oxidized. – The Cu2+ gained 2 electrons, so it was reduced.
- 470. • Fe (metal) + Cu2+ Fe2+ + Cu (metal) – Fe donates two electrons to the Cu2+ to form Cu (metal). The Fe lost 2 electrons, so it was oxidized. – The Cu2+ gained 2 electrons, so it was reduced.
- 472. • Fe2O3 + 3 CO 2 Fe + 3 CO2
- 473. • Fe2O3 + 3 CO 2 Fe + 3 CO2
- 474. • Fe2O3 + 3 CO 2 Fe + 3 CO2 Oxidation is loss of oxygen. Oxidation is gain of oxygen.
- 475. • Fe2O3 + 3 CO 2 Fe + 3 CO2 Oxidation is loss of oxygen. Reduction is gain of oxygen.
- 476. • Fe2O3 + 3 CO 2 Fe + 3 CO2 Reduction is loss of oxygen. Oxidation is gain of oxygen.
- 477. CO + H2O CO2 + H2 +2 +1 +4 +0 Oxidation is loss of oxygen. Reduction is gain of oxygen.
- 478. CO + H2O CO2 + H2 +2 +1 +4 +0 Oxidation is loss of oxygen. Reduction is gain of oxygen.
- 479. CO + H2O CO2 + H2 +2 +1 +4 +0 Oxidation is loss of oxygen. Reduction is gain of oxygen.
- 480. CO + H2O CO2 + H2 +2 +1 +4 +0 Oxidation is loss of oxygen. Reduction is gain of oxygen.
- 481. CO + H2O CO2 + H2 +2 +1 +4 +0 Oxidation is loss of oxygen. Reduction is gain of oxygen.
- 483. 2 Al(s) + Fe2O3(s) Al2O3(s) + 2 Fe(l)
- 484. 2 Al(s) + Fe2O3(s) Al2O3(s) + 2 Fe(l)
- 485. 2 Al(s) + Fe2O3(s) Al2O3(s) + 2 Fe(l) 0 +3 +3 +O Oxidation is loss of oxygen. Reduction is gain of oxygen.
- 486. 2 Al(s) + Fe2O3(s) Al2O3(s) + 2 Fe(l) 0 +3 +3 +O Oxidation is loss of oxygen. Reduction is gain of oxygen.
- 487. 2 Al(s) + Fe2O3(s) Al2O3(s) + 2 Fe(l) 0 +3 +3 +O Oxidation is loss of oxygen. Reduction is gain of oxygen.
- 488. 2 Al(s) + Fe2O3(s) Al2O3(s) + 2 Fe(l) 0 +3 +3 +O Oxidation is loss of oxygen. Reduction is gain of oxygen.
- 489. • There’s a lot more to learn about oxidation and reduction reactions. • Learn more… http://www.shodor.org/unchem/advanced/redox/index.html • http://iit.edu/arc/workshops/pdfs/RedOx_Rxns.pdf • http://hyperphysics.phy- astr.gsu.edu/hbase/chemical/oxred.html • http://www.chemtutor.com/redox.htm • http://chemwiki.ucdavis.edu/Analytical_Chemistry/Electrochemi stry/Redox_Chemistry/Oxidizing_and_Reducing_Agents • Assigning Oxidation Numbers – https://www.youtube.com/watch?v=w0RfMRDy34w
- 490. • Video Link! Hank explains how oxygen is killing us. – http://www.youtube.com/watch?v=VnAhAX98HY4
- 491. • Video Link! Redox Crash Course. – Advanced and Optional – http://www.youtube.com/watch?v=lQ6FBA1HM3s &list=PL8dPuuaLjXtPHzzYuWy6fYEaX9mQQ8o Gr
- 492. • Video Link! Khan Academy Oxidation and Reduction (Optional / Advanced) – https://www.youtube.com/watch?v=orI2m6IarJg
- 493. • Try and be the first to name the picture hidden beneath the boxes? – Raise your hand when you think you know. You only get one guess. – These two box games are work bonus points on the balancing chemical equations sheet. Copyright © 2010 Ryan P. Murphy
- 494. “Covalent Bond.” Copyright © 2010 Ryan P. Murphy
- 495. “Covalent Bond.” Copyright © 2010 Ryan P. Murphy
- 496. “Covalent Bond.” Copyright © 2010 Ryan P. Murphy
- 497. “Covalent Bond.” Copyright © 2010 Ryan P. Murphy
- 498. “Covalent Bond.” Copyright © 2010 Ryan P. Murphy
- 499. “Covalent Bond.” Copyright © 2010 Ryan P. Murphy
- 500. “Covalent Bond.” Copyright © 2010 Ryan P. Murphy
- 501. “Covalent Bond.” Copyright © 2010 Ryan P. Murphy
- 502. • Try Again! Can you name the picture hidden beneath the boxes? – Raise your hand when you think you know. You only get one guess. – These two box games are work bonus points on the balancing chemical equations sheet. Copyright © 2010 Ryan P. Murphy
- 521. • Try Again! Can you name the picture hidden beneath the boxes? – Raise your hand when you think you know. You only get one guess. – These two box games are work bonus points on the balancing chemical equations sheet. Copyright © 2010 Ryan P. Murphy
- 522. Copyright © 2010 Ryan P. Murphy
- 523. Copyright © 2010 Ryan P. Murphy
- 524. Copyright © 2010 Ryan P. Murphy
- 525. Copyright © 2010 Ryan P. Murphy
- 526. Copyright © 2010 Ryan P. Murphy
- 527. Copyright © 2010 Ryan P. Murphy
- 528. Copyright © 2010 Ryan P. Murphy
- 529. Copyright © 2010 Ryan P. Murphy
- 530. Copyright © 2010 Ryan P. Murphy
- 531. • Try Again! Can you name the picture hidden beneath the boxes? – Raise your hand when you think you know. You only get one guess. – These two box games are work bonus points on the balancing chemical equations sheet. Copyright © 2010 Ryan P. Murphy
- 548. • Please complete this page in your bundle
