States of Matter and Phase Change, Physical Science Lesson PowerPoint
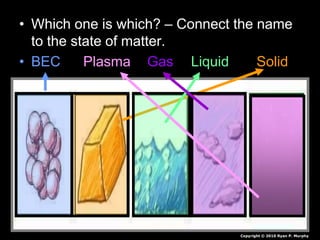
- 1. • Which one is which? – Connect the name to the state of matter. • BEC Plasma Gas Liquid Solid Copyright © 2010 Ryan P. Murphy
- 3. • RED SLIDE: These are notes that are very important and should be recorded in your science journal. Copyright © 2010 Ryan P. Murphy
- 4. -Nice neat notes that are legible and use indentations when appropriate. -Example of indent. -Skip a line between topics -Don’t skip pages -Make visuals clear and well drawn. Please label. Ice Melting Water Boiling Vapor GasT E M P Heat Added
- 5. • RED SLIDE: These are notes that are very important and should be recorded in your science journal. • BLACK SLIDE: Pay attention, follow directions, complete projects as described and answer required questions neatly. Copyright © 2010 Ryan P. Murphy
- 7. Matter, Energy, and the Environment Unit Copyright © 2010 Ryan P. Murphy
- 8. First Area of Focus: Matter
- 9. Matter : Anything that has mass and takes up space. Copyright © 2010 Ryan P. Murphy
- 10. Matter : Anything that has mass and takes up space. Copyright © 2010 Ryan P. Murphy
- 11. Element: A substance that is made entirely from one type of atom. Copyright © 2010 Ryan P. Murphy
- 12. Compound: Made up of two or more elements bonded together. Copyright © 2010 Ryan P. Murphy
- 21. Homogeneous: Composed of elements that are all the same.
- 24. Heterogeneous / Inhomogeneous: Composed of two or more different types of elements.
- 26. • Which picture below best represents a homogeneous mixture, and which represents a heterogeneous mixture?
- 27. • Which picture below best represents a homogeneous mixture, and which represents a heterogeneous mixture?
- 28. • Which picture below best represents a homogeneous mixture, and which represents a heterogeneous mixture?
- 29. • Which picture below best represents a homogeneous mixture, and which represents a heterogeneous mixture?
- 30. • Which picture below best represents a homogeneous mixture, and which represents a heterogeneous mixture?
- 31. • Which picture below best represents a homogeneous mixture, and which represents a heterogeneous mixture?
- 32. • Which picture below best represents a homogeneous mixture, and which represents a heterogeneous mixture?
- 33. • Which picture below best represents a homogeneous mixture, and which represents a heterogeneous mixture?
- 34. • Which picture below best represents a homogeneous mixture, and which represents a heterogeneous mixture?
- 35. • Which picture below best represents a homogeneous mixture, and which represents a heterogeneous mixture? Learn More about mixtures: http://www.elmhurst.edu/~chm/vchembook/106Amixture.html
- 36. Law Conservation of Matter - Copyright © 2010 Ryan P. Murphy
- 37. In any physical or chemical change, matter is neither created nor destroyed Copyright © 2010 Ryan P. Murphy
- 38. In any physical or chemical change, matter is neither created nor destroyed Matter can be changed from one form to another. Copyright © 2010 Ryan P. Murphy
- 39. In any physical or chemical change, matter is neither created nor destroyed Matter can be changed from one form to another. Copyright © 2010 Ryan P. Murphy
- 40. In any physical or chemical change, matter is neither created nor destroyed Matter can be changed from one form to another. Copyright © 2010 Ryan P. Murphy
- 41. In any physical or chemical change, matter is neither created nor destroyed Matter can be changed from one form to another. Copyright © 2010 Ryan P. Murphy
- 42. In any physical or chemical change, matter is neither created nor destroyed Matter can be changed from one form to another. Copyright © 2010 Ryan P. Murphy
- 43. In any physical or chemical change, matter is neither created nor destroyed Matter can be changed from one form to another. Copyright © 2010 Ryan P. Murphy
- 44. In any physical or chemical change, matter is neither created nor destroyed Matter can be changed from one form to another. Copyright © 2010 Ryan P. Murphy
- 45. In any physical or chemical change, matter is neither created nor destroyed Matter can be changed from one form to another. Copyright © 2010 Ryan P. Murphy
- 47. Big Bang All Matter Particles join together
- 48. Big Bang All Matter Particles join together Gravity attracts particles, forms stars, planets Galaxies
- 49. Big Bang All Matter Particles join together Gravity attracts particles, forms stars, planets Galaxies Sun releases particles, photons through nuclear processes
- 50. Big Bang All Matter Particles join together Gravity attracts particles, forms stars, planets Galaxies Sun releases particles, photons through nuclear processes Plants harness Photons to make sugars with available molecules on Earth from formation
- 51. Big Bang All Matter Particles join together Gravity attracts particles, forms stars, planets Galaxies Sun releases particles, photons through nuclear processes Plants harness Photons to make sugars with available molecules on Earth from formation
- 52. Big Bang All Matter Particles join together Gravity attracts particles, forms stars, planets Galaxies Sun releases particles, photons through nuclear processes Plants harness Photons to make sugars with available molecules on Earth from formation Matter from the formation of the planets, sometime after the big bang.
- 53. Big Bang All Matter Particles join together Gravity attracts particles, forms stars, planets Galaxies Sun releases particles, photons through nuclear processes Plants harness Photons to make sugars with available molecules on Earth from formation Matter from the formation of the planets, sometime after the big bang.
- 54. Big Bang All Matter Particles join together Gravity attracts particles, forms stars, planets Galaxies Sun releases particles, photons through nuclear processes Plants harness Photons to make sugars with available molecules on Earth from formation Matter from the formation of the planets, sometime after the big bang.
- 55. Big Bang All Matter Particles join together Gravity attracts particles, forms stars, planets Galaxies Sun releases particles, photons through nuclear processes Plants harness Photons to make sugars with available molecules on Earth from formation Matter from the formation of the planets, sometime after the big bang.
- 56. • Available Worksheet: Law Conservation of Mass.
- 57. • Available Worksheet: Law Conservation of Mass.
- 58. • Activity! Law Conservation of Mass – Secure a birthday candle to a Petri-Dish and weigh all. – Light candle on a scale and record the weight of the candle every minute for 10 minutes. • Light fan can speed combustion / results. – Spreadsheet on next page. – Lab questions to be answered at end. Copyright © 2010 Ryan P. Murphy
- 59. • Record the Following Spreadsheet into your Journal. Time Weight of Candle at Start (grams) Start 5 10 15 20 25 30 35 40 45 50 Please graph results in a line graph. Copyright © 2010 Ryan P. Murphy
- 60. grams grams grams grams grams Copyright © 2010 Ryan P. Murphy
- 61. 5 grams 4 grams 3 grams 2 grams 1 gram Copyright © 2010 Ryan P. Murphy -Simulated data if not conducting demonstration
- 62. • Questions! Copyright © 2010 Ryan P. Murphy
- 63. • Questions! – Why did the candle decrease in mass? Copyright © 2010 Ryan P. Murphy
- 64. • Questions! – Why did the candle decrease in mass? – Did the flame destroy matter (candle) or just change its form? Copyright © 2010 Ryan P. Murphy
- 65. • Questions! – Why did the candle decrease in mass? – Did the flame destroy matter (candle) or just change its form? – From what form did the candle change? Copyright © 2010 Ryan P. Murphy
- 66. • Questions! – Why did the candle decrease in mass? – Did the flame destroy matter (candle) or just change its form? – From what form did the candle change? Copyright © 2010 Ryan P. Murphy
- 67. • Answers to Questions! Copyright © 2010 Ryan P. Murphy
- 68. • Answers to Questions! – Why did the candle decrease in mass? Copyright © 2010 Ryan P. Murphy
- 69. • Questions! – Why did the candle decrease in mass? – Answer! Because the candle which was a solid turned into a gas during combustion. The gas was not collected to be measured. Copyright © 2010 Ryan P. Murphy
- 70. • Questions! – Did the flame destroy matter (candle) or just change its form? Copyright © 2010 Ryan P. Murphy
- 71. • Questions! – Did the flame destroy matter (candle) or just change its form? – Answer! No, Matter cannot be created or destroyed but changed from one form to another.
- 72. • Questions! – From what form did the candle change? Copyright © 2010 Ryan P. Murphy
- 73. • Questions! – From what form did the candle change? – Answer! The candle changed from a solid to a liquid (melting) and into a gas (evaporation). Copyright © 2010 Ryan P. Murphy
- 78. • Demonstration of Law Conservation of Matter. – Weigh Alka-Seltzer and water solution tablet in grams _____ – Weigh 100 ml of water in grams ______ – Pour water into large zip-lock bag. • Predict the mass if we add Alka-Seltzer to the water bag and immediately seal the bag. • Predict the mass if we add Alka-Seltzer to the water bag and don’t seal the bag. Copyright © 2010 Ryan P. Murphy
- 79. • Demonstration of Law Conservation of Matter. – Weigh Alka-Seltzer and water solution tablet in grams _____ – Weigh 100 ml of water in grams ______ – Pour water into large zip-lock bag. • Predict the mass if we add Alka-Seltzer to the water bag and immediately seal the bag. • Predict the mass if we add Alka-Seltzer to the water bag and don’t seal the bag. Copyright © 2010 Ryan P. Murphy
- 80. • Demonstration of Law Conservation of Matter. – Weigh Alka-Seltzer and water solution tablet in grams _____ – Weigh 100 ml of water in grams ______ – Pour water into large zip-lock bag. • Predict the mass if we add Alka-Seltzer to the water bag and immediately seal the bag. • Predict the mass if we add Alka-Seltzer to the water bag and don’t seal the bag. Copyright © 2010 Ryan P. Murphy
- 81. • Demonstration of Law Conservation of Matter. – Weigh Alka-Seltzer and water solution tablet in grams _____ – Weigh 100 ml of water in grams ______ – Pour water into sandwich size Zip-Lock bag. • Predict the mass if we add Alka-Seltzer to the water bag and immediately seal the bag. • Predict the mass if we add Alka-Seltzer to the water bag and don’t seal the bag. Copyright © 2010 Ryan P. Murphy
- 82. • Demonstration of Law Conservation of Matter. – Weigh Alka-Seltzer and water solution tablet in grams _____ – Weigh 100 ml of water in grams ______ – Pour water into sandwich size Zip-Lock bag. • Predict the mass if we add Alka-Seltzer to the water bag and immediately seal the bag. • Predict the mass if we add Alka-Seltzer to the water bag and don’t seal the bag. Copyright © 2010 Ryan P. Murphy
- 83. • Demonstration of Law Conservation of Matter. – Weigh Alka-Seltzer and water solution tablet in grams _____ – Weigh 100 ml of water in grams ______ – Pour water into sandwich size Zip-Lock bag. • Predict the mass if we add Alka-Seltzer to the water bag and immediately seal the bag. • Predict the mass if we add Alka-Seltzer to the water bag and don’t seal the bag. Copyright © 2010 Ryan P. Murphy
- 84. • Demonstration of Law Conservation of Matter. – Weight of water _____? – Weight of Alka-Seltzer _____? – Weight together in sealed bag _____? – Weight together in unsealed bag _____? Copyright © 2010 Ryan P. Murphy
- 85. • Demonstration of Law Conservation of Matter Questions. – What happened when the two mixed? – Why was the weight of the water and tablet combined in the sealed bag the same as them separate? – Why did unsealing the bag decrease the weight of the two together. Copyright © 2010 Ryan P. Murphy
- 86. • Demonstration of Law Conservation of Matter Questions. – What happened when the two mixed? – Why was the weight of the water and tablet combined in the sealed bag the same as them separate? – Why did unsealing the bag decrease the weight of the two together. Copyright © 2010 Ryan P. Murphy
- 87. • Demonstration of Law Conservation of Matter Questions. – What happened when the two mixed? – Why was the weight of the water and tablet combined in the sealed bag the same as them separate? – Why did unsealing the bag decrease the weight of the two together. Copyright © 2010 Ryan P. Murphy
- 88. • Demonstration of Law Conservation of Matter Questions. – What happened when the two mixed? – Why was the weight of the water and tablet combined in the sealed bag the same as them separate? – Why did unsealing the bag decrease the weight of the two together? Copyright © 2010 Ryan P. Murphy
- 89. • Demonstration of Law Conservation of Matter Questions. Copyright © 2010 Ryan P. Murphy
- 90. • Demonstration of Law Conservation of Matter Questions. – What happened when the two mixed? Copyright © 2010 Ryan P. Murphy
- 91. • Demonstration of Law Conservation of Matter Questions. – What happened when the two mixed? – Answer! The Alka-Seltzer reacted with the water and released a gas (carbon dioxide). Copyright © 2010 Ryan P. Murphy
- 92. • Demonstration of Law Conservation of Matter Questions. Copyright © 2010 Ryan P. Murphy
- 93. • Demonstration of Law Conservation of Matter Questions. – Why was the weight of the water and tablet combined in the sealed bag the same as them separate? Copyright © 2010 Ryan P. Murphy
- 94. • Demonstration of Law Conservation of Matter Questions. – Why was the weight of the water and tablet combined in the sealed bag the same as them separate? – Answer! Law Conservation of Matter. No gas was allowed to escape. Copyright © 2010 Ryan P. Murphy
- 95. • Demonstration of Law Conservation of Matter Questions. Copyright © 2010 Ryan P. Murphy
- 96. • Demonstration of Law Conservation of Matter Questions. – Why did unsealing the bag decrease the weight of the two together? Copyright © 2010 Ryan P. Murphy
- 97. • Demonstration of Law Conservation of Matter Questions. – Why did unsealing the bag decrease the weight of the two together? – Answer! The carbon dioxide gas was allowed to escape into the air which wasn’t recorded mass. Copyright © 2010 Ryan P. Murphy
- 98. • Demonstration of Law Conservation of Matter Questions. – Why did unsealing the bag decrease the weight of the two together? – Answer! The carbon dioxide gas was allowed to escape into the air which wasn’t recorded mass. – Optional Class Quiz found at • http://home.utah.edu/~u0577548 /Conservation%20of%20Matter/s um_of_parts_quiz.html Copyright © 2010 Ryan P. Murphy
- 99. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy
- 100. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy
- 101. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy
- 102. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy What are the states of matter?
- 103. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy
- 104. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy
- 105. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy
- 106. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy
- 107. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy
- 108. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy
- 109. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy
- 110. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy
- 111. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy
- 112. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy
- 113. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy
- 114. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy
- 115. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy
- 116. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy
- 117. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy
- 118. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy
- 119. Kinetic Molecular Theory: The molecules are in constant motion. This motion is different for the 3 states of matter. Copyright © 2010 Ryan P. Murphy Kinetic Molecular Theory. Learn More: http://www.chm.davidson.edu/vce/kineticmolecularth eory/basicconcepts.
- 120. • Activity! State your Matter – Teacher to give each group of students a solid block (Maybe ice), glass of water, and balloon filled with gas.
- 121. • Activity! State your Matter – Teacher to give each group of students a solid block (Maybe ice), glass of water, and balloon filled with gas.
- 122. • Activity! State your Matter – Teacher to give each group of students a solid block (Maybe ice), glass of water, and balloon filled with gas.
- 123. • Activity! State your Matter – Teacher to give each group of students a solid block (Maybe ice), glass of water, and balloon filled with gas.
- 124. • Activity Sheet Available: States of Matter and Phase Change.
- 125. • Activity Sheet Available: States of Matter and Phase Change.
- 126. • Activity! Describing Solid-Liquid-Gas – Please fill out the following spreadsheet and then collect data. – Find it or write (?) Solid Liquid Gas Volume L*W*H Shape Mass Copyright © 2010 Ryan P. Murphy
- 127. • Activity! Describing Solid-Liquid-Gas – Possible Answers! Solid Liquid Gas Volume Easy to find – in ml or cm3 Easy to find. Use graduated cylinder – ml Difficult to find in a classroom . Shape Many different forms. Easy to mold. Takes shape of the container. No Shape Mass Generally Heavy / Weigh in grams Easy to find. Generally Heavy / Weigh in grams. Lighter in mass / Harder to weigh in a classroom Copyright © 2010 Ryan P. Murphy
- 128. • Activity! Describing Solid-Liquid-Gas – Possible Answers! Solid Liquid Gas Volume Easy to find – in ml or cm3 Easy to find. Use graduated cylinder – ml Difficult to find in a classroom . Shape Many different forms. Easy to mold. Takes shape of the container. No Shape Mass Generally Heavy / Weigh in grams Easy to find. Generally Heavy / Weigh in grams. Lighter in mass / Harder to weigh in a classroom Copyright © 2010 Ryan P. Murphy
- 129. • Activity! Describing Solid-Liquid-Gas – Possible Answers! Solid Liquid Gas Volume Easy to find – in ml or cm3 Easy to find. Use graduated cylinder – ml Difficult to find in a classroom . Shape Many different forms. Easy to mold. Takes shape of the container. No Shape Mass Generally Heavy / Weigh in grams Easy to find. Generally Heavy / Weigh in grams. Lighter in mass / Harder to weigh in a classroom Copyright © 2010 Ryan P. Murphy
- 130. • Activity! Describing Solid-Liquid-Gas – Possible Answers! Solid Liquid Gas Volume Easy to find – in ml or cm3 Easy to find. Use graduated cylinder – ml Difficult to find in a classroom . Shape Many different forms. Easy to mold. Takes shape of the container. No Shape Mass Generally Heavy / Weigh in grams Easy to find. Generally Heavy / Weigh in grams. Lighter in mass / Harder to weigh in a classroom Copyright © 2010 Ryan P. Murphy
- 131. • Activity! Describing Solid-Liquid-Gas – Possible Answers! Solid Liquid Gas Volume Easy to find – in ml or cm3 Easy to find. Use graduated cylinder – ml Difficult to find in a classroom . Shape Many different forms. Easy to mold. Takes shape of the container. No Shape Mass Generally Heavy / Weigh in grams Easy to find. Generally Heavy / Weigh in grams. Lighter in mass / Harder to weigh in a classroom Copyright © 2010 Ryan P. Murphy
- 132. • Activity! Describing Solid-Liquid-Gas – Possible Answers! Solid Liquid Gas Volume Easy to find – in ml or cm3 Easy to find. Use graduated cylinder – ml Difficult to find in a classroom . Shape Many different forms. Easy to mold. Takes shape of the container. No Shape Mass Generally Heavy / Weigh in grams Easy to find. Generally Heavy / Weigh in grams. Lighter in mass / Harder to weigh in a classroom Copyright © 2010 Ryan P. Murphy
- 133. • Activity! Describing Solid-Liquid-Gas – Possible Answers! Solid Liquid Gas Volume Easy to find – in ml or cm3 Easy to find. Use graduated cylinder – ml Difficult to find in a classroom . Shape Many different forms. Easy to mold. Takes shape of the container. No Shape Mass Generally Heavy / Weigh in grams Easy to find. Generally Heavy / Weigh in grams. Lighter in mass / Harder to weigh in a classroom Copyright © 2010 Ryan P. Murphy
- 134. • Activity! Describing Solid-Liquid-Gas – Possible Answers! Solid Liquid Gas Volume Easy to find – in ml or cm3 Easy to find. Use graduated cylinder – ml Difficult to find in a classroom . Shape Many different forms. Easy to mold. Takes shape of the container. No Shape Mass Generally Heavy / Weigh in grams Easy to find. Generally Heavy / Weigh in grams. Lighter in mass / Harder to weigh in a classroom Copyright © 2010 Ryan P. Murphy
- 135. • Activity! Describing Solid-Liquid-Gas – Possible Answers! Solid Liquid Gas Volume Easy to find – in ml or cm3 Easy to find. Use graduated cylinder – ml Difficult to find in a classroom . Shape Many different forms. Easy to mold. Takes shape of the container. No Shape Mass Generally Heavy / Weigh in grams Easy to find. Generally Heavy / Weigh in grams. Lighter in mass / Harder to weigh in a classroom Copyright © 2010 Ryan P. Murphy
- 136. • Activity! Describing Solid-Liquid-Gas – Possible Answers! Solid Liquid Gas Volume Easy to find – in ml or cm3 Easy to find. Use graduated cylinder – ml Difficult to find in a classroom . Shape Many different forms. Easy to mold. Takes shape of the container. No Shape Mass Generally Heavy / Weigh in grams Easy to find. Generally Heavy / Weigh in grams. Lighter in mass / Harder to weigh in a classroom Copyright © 2010 Ryan P. Murphy
- 137. • Activity! Describing Solid-Liquid-Gas – Possible Answers! Solid Liquid Gas Volume Easy to find – in ml or cm3 Easy to find. Use graduated cylinder – ml Difficult to find in a classroom . Shape Many different forms. Easy to mold. Takes shape of the container. No Shape Mass Generally Heavy / Weigh in grams Easy to find. Generally Heavy / Weigh in grams. Lighter in mass / Harder to weigh in a classroom Copyright © 2010 Ryan P. Murphy
- 138. • Activity! Describing Solid-Liquid-Gas – Possible Answers! Solid Liquid Gas Volume Easy to find – in ml or cm3 Easy to find. Use graduated cylinder – ml Difficult to find in a classroom . Shape Many different forms. Easy to mold. Takes shape of the container. No Shape Mass Generally Heavy / Weigh in grams Easy to find. Generally Heavy / Weigh in grams. Lighter in mass / Harder to weigh in a classroom Copyright © 2010 Ryan P. Murphy
- 139. • Activity! Describing Solid-Liquid-Gas – Possible Answers! Solid Liquid Gas Volume Easy to find – in ml or cm3 Easy to find. Use graduated cylinder – ml Difficult to find in a classroom . Shape Many different forms. Easy to mold. Takes shape of the container. No Shape Mass Generally Heavy / Weigh in grams Easy to find. Generally Heavy / Weigh in grams. Lighter in mass / Harder to weigh in a classroom Copyright © 2010 Ryan P. Murphy
- 140. • Activity! Describing Solid-Liquid-Gas – Possible Answers! Solid Liquid Gas Volume Easy to find – in ml or cm3 Easy to find. Use graduated cylinder – ml Difficult to find in a classroom . Shape Many different forms. Easy to mold. Takes shape of the container. No Shape Mass Generally Heavy / Weigh in grams Easy to find. Generally Heavy / Weigh in grams. Lighter in mass / Harder to weigh in a classroom Copyright © 2010 Ryan P. Murphy
- 141. • Activity! Describing Solid-Liquid-Gas – Possible Answers! Solid Liquid Gas Volume Easy to find – in ml or cm3 Easy to find. Use graduated cylinder – ml Difficult to find in a classroom . PV=nRT Shape Many different forms. Easy to mold. Takes shape of the container. No Shape Mass Generally Heavy / Weigh in grams Easy to find. Generally Heavy / Weigh in grams. Lighter in mass / Harder to weigh in a classroom Copyright © 2010 Ryan P. Murphy
- 142. • Activity! Describing Solid-Liquid-Gas – Possible Answers! Solid Liquid Gas Volume Easy to find – in ml or cm3 Easy to find. Use graduated cylinder – ml Difficult to find in a classroom . Shape Many different forms. Easy to mold. Takes shape of the container. No Shape Mass Generally Heavy / Weigh in grams Easy to find. Generally Heavy / Weigh in grams. Lighter in mass / Harder to weigh in a classroom Copyright © 2010 Ryan P. Murphy
- 143. • Activity! Describing Solid-Liquid-Gas – Possible Answers! Solid Liquid Gas Volume Easy to find – in ml or cm3 Easy to find. Use graduated cylinder – ml Difficult to find in a classroom . Shape Many different forms. Easy to mold. Takes shape of the container. No Shape Mass Generally Heavy / Weigh in grams Easy to find. Generally Heavy / Weigh in grams. Lighter in mass / Harder to weigh in a classroom Copyright © 2010 Ryan P. Murphy
- 144. • Activity! Describing Solid-Liquid-Gas – Possible Answers! Solid Liquid Gas Volume Easy to find – in ml or cm3 Easy to find. Use graduated cylinder – ml Difficult to find in a classroom . Shape Many different forms. Easy to mold. Takes shape of the container. No Shape Mass Generally Heavy / Weigh in grams Easy to find. Generally Heavy / Weigh in grams. Lighter in mass / Harder to weigh in a classroom Copyright © 2010 Ryan P. Murphy
- 145. • Activity! Describing Solid-Liquid-Gas – Possible Answers! Solid Liquid Gas Volume Easy to find – in ml or cm3 Easy to find. Use graduated cylinder – ml Difficult to find in a classroom . Shape Many different forms. Easy to mold. Takes shape of the container. No Shape Mass Generally Heavy / Weigh in grams Easy to find. Generally Heavy / Weigh in grams. Lighter in mass / Harder to weigh than solid and liquids. Copyright © 2010 Ryan P. Murphy
- 146. • Activity! State your Matter – Teacher to give each group of students a solid block (Maybe ice), glass of water, and balloon filled with gas.
- 147. • Activity! State your Matter – Teacher to give each group of students a solid block (Maybe ice), glass of water, and balloon filled with gas.
- 148. • Activity! State your Matter – Teacher to give each group of students a solid block (Maybe ice), glass of water, and balloon filled with gas.
- 149. • Activity! State your Matter – Teacher to give each group of students a solid block (Maybe ice), glass of water, and balloon filled with gas.
- 150. • Activity! State your Matter – Teacher to give each group of students a solid block (Maybe ice), glass of water, and balloon filled with gas.
- 151. • Activity! State your Matter – Teacher to give each group of students a solid block (Maybe ice), glass of water, and balloon filled with gas.
- 152. • Activity! State your Matter – Teacher to give each group of students a solid block (Maybe ice), glass of water, and balloon filled with gas.
- 153. • Activity! State your Matter – Teacher to give each group of students a solid block (Maybe ice), glass of water, and balloon filled with gas.
- 154. • Activity! State your Matter – Teacher to give each group of students a solid block (Maybe ice), glass of water, and balloon filled with gas.
- 155. • Activity! State your Matter – Teacher to give each group of students a solid block (Maybe ice), glass of water, and balloon filled with gas.
- 156. • Activity! State your Matter – Teacher to give each group of students a solid block (Maybe ice), glass of water, and balloon filled with gas.
- 157. States of Matter - - - - Copyright © 2010 Ryan P. Murphy
- 158. Solid (s) has a definite shape and volume. Copyright © 2010 Ryan P. Murphy
- 163. • Activity! Semi-Solid • Diapers contain polyacrylic acid, a super- absorbent polymer (large molecule). – http://www.coolscience.org/CoolScience/KidScie ntists/babydiaper.htm (Learn More) – This molecule is hydophilic (water loving)
- 164. • Activity! Semi-Solid • Diapers contain polyacrylic acid, a super- absorbent polymer (large molecule). – http://www.coolscience.org/CoolScience/KidScie ntists/babydiaper.htm (Learn More) – This molecule is hydophilic (water loving)
- 165. • Activity! Semi-Solid • Diapers contain polyacrylic acid, a super- absorbent polymer (large molecule). – http://www.coolscience.org/CoolScience/KidScie ntists/babydiaper.htm (Learn More) – This molecule is hydophilic (water loving)
- 166. • Activity! Semi-Solid • Diapers contain polyacrylic acid, a super- absorbent polymer (large molecule). – http://www.coolscience.org/CoolScience/KidScie ntists/babydiaper.htm (Learn More) – This molecule is hydophilic (water loving)
- 167. • Activity! Semi-Solid • Diapers contain polyacrylic acid, a super- absorbent polymer (large molecule). – http://www.coolscience.org/CoolScience/KidScie ntists/babydiaper.htm (Learn More) – This molecule is hydophilic (water loving)
- 168. • Activity! Semi-Solid • Diapers contain polyacrylic acid, a super- absorbent polymer (large molecule). – http://www.coolscience.org/CoolScience/KidScie ntists/babydiaper.htm (Learn More) – This molecule is hydophilic (water loving)
- 170. • Semi-solid. While similar to a solid in some respects (it can support its own weight and hold its shape), it also shares some properties of liquids, such as shape conformity to something applying pressure to it, or the ability to flow under pressure.
- 171. Liquid (l) Has definite volume but not shape. Copyright © 2010 Ryan P. Murphy
- 175. Gas (g) No definite shape or volume. Copyright © 2010 Ryan P. Murphy
- 176. Gas (g) No definite shape or volume. Copyright © 2010 Ryan P. Murphy
- 180. • Activity / video link (Extremely Optional) – http://www.youtube.com/watch?v=p440QWpHui8 – Assign three students to each hold a poster with the three states of matter. (Solid, Liquid, Gas) • When Zebra is dancing and singing fast person with gas poster must wave it around quickly. • When zebra is dancing normal wave the liquid poster at a normal speed. • When zebra is dancing slowly wave the solid poster extremely slow.
- 181. • Activity / video link (Extremely Optional) – http://www.youtube.com/watch?v=p440QWpHui8 – Assign three students to each hold a poster with the three states of matter. (Solid, Liquid, Gas) • When Zebra is dancing at a fast pace the person with gas poster must wave it around quickly / dance. • When zebra is dancing normal wave the liquid poster at a normal speed / slower dance. • When zebra is dancing slowly wave the solid poster extremely slow / slow dance.
- 182. • Based on the video, which is a solid, liquid, and gas.
- 183. • Based on the video, which is a solid, liquid, and gas.
- 184. • Based on the video, which is a solid, liquid, and gas.
- 185. • Based on the video, which is a solid, liquid, and gas.
- 186. • Based on the video, which is a solid, liquid, and gas.
- 187. • Based on the video, which is a solid, liquid, and gas.
- 188. • Based on the video, which is a solid, liquid, and gas.
- 189. • Based on the video, which is a solid, liquid, and gas.
- 190. • Based on the video, which is a solid, liquid, and gas.
- 191. • Based on the video, which is a solid, liquid, and gas.
- 192. • Based on the video, which is a solid, liquid, and gas.
- 193. • Activity / video link (Extremely Optional) – http://www.youtube.com/watch?v=p440QWpHui8 – Assign three students to each hold a poster with the three states of matter. (Solid, Liquid, Gas) • When Zebra is dancing and singing fast person with gas poster must wave it around quickly. • When zebra is dancing normal wave the liquid poster at a normal speed. • When zebra is dancing slowly wave the solid poster extremely slow.
- 194. • Activity / video link (Extremely Optional) – http://www.youtube.com/watch?v=p440QWpHui8 – Assign three students to each hold a poster with the three states of matter. (Solid, Liquid, Gas) • When Zebra is dancing and singing fast person with gas poster must wave it around quickly. • When zebra is dancing normal wave the liquid poster at a normal speed. • When zebra is dancing slowly wave the solid poster extremely slow.
- 195. • Activity / video link (Extremely Optional) – http://www.youtube.com/watch?v=p440QWpHui8 – Assign three students to each hold a poster with the three states of matter. (Solid, Liquid, Gas) • When Zebra is dancing and singing fast person with gas poster must wave it around quickly. • When zebra is dancing normal wave the liquid poster at a normal speed. • When zebra is dancing slowly wave the solid poster extremely slow.
- 196. • Activity / video link (Extremely Optional) – http://www.youtube.com/watch?v=p440QWpHui8 – Assign three students to each hold a poster with the three states of matter. (Solid, Liquid, Gas) • When Zebra is dancing and singing fast person with gas poster must wave it around quickly. • When zebra is dancing normal wave the liquid poster at a normal speed. • When zebra is dancing slowly wave the solid poster extremely slow.
- 197. • Activity / video link (Extremely Optional) – http://www.youtube.com/watch?v=p440QWpHui8 – Assign three students to each hold a poster with the three states of matter. (Solid, Liquid, Gas) • When Zebra is dancing and singing fast person with gas poster must wave it around quickly. • When zebra is dancing normal wave the liquid poster at a normal speed. • When zebra is dancing slowly wave the solid poster extremely slow.
- 198. • Activity / video link (Extremely Optional) – http://www.youtube.com/watch?v=p440QWpHui8 – Assign three students to each hold a poster with the three states of matter. (Solid, Liquid, Gas) • When Zebra is dancing and singing fast person with gas poster must wave it around quickly. • When zebra is dancing normal wave the liquid poster at a normal speed. • When zebra is dancing slowly wave the solid poster extremely slow.
- 199. • Activity / video link (Extremely Optional) – http://www.youtube.com/watch?v=p440QWpHui8 – Assign three students to each hold a poster with the three states of matter. (Solid, Liquid, Gas) • When Zebra is dancing and singing fast person with gas poster must wave it around quickly. • When zebra is dancing normal wave the liquid poster at a normal speed. • When zebra is dancing slowly wave the solid poster extremely slow.
- 200. • Video Link! (Optional) TMBG – http://www.youtube.com/watch?v=btGu9FWSPtc
- 201. • Diffusion: Random movement of molecules. – From high to low concentrations. Copyright © 2010 Ryan P. Murphy
- 202. • Diffusion: Random movement of molecules. – From high to low concentrations. Copyright © 2010 Ryan P. Murphy
- 203. • Why do substances always flow from high concentrations to low concentrations? Copyright © 2010 Ryan P. Murphy
- 204. • Answer! Kinetic movement of molecules causes particles to move to open areas. Copyright © 2010 Ryan P. Murphy
- 205. Copyright © 2010 Ryan P. Murphy
- 206. • Heat Diffusion through a room.
- 207. • Activity! Making the room smell good. – Teacher to stand in one place and release some spray. – Raise your hand when you smell it. – What are the molecules doing? Copyright © 2010 Ryan P. Murphy
- 208. • Answer: The Molecules are trying to reach equilibrium. Copyright © 2010 Ryan P. Murphy
- 209. • What is the fourth state of matter?
- 210. Plasma (p) Ionized gas that emits electrons. Copyright © 2010 Ryan P. Murphy
- 212. • 99.9% of normal matter is Plasma.
- 213. • 99.9% of normal matter is Plasma. STARS
- 214. • 99.9% of normal matter is Plasma. STARS – So that .1% is the (s),(l),(g) that we are made of.
- 215. • BEC’s
- 216. • A Bose–Einstein condensate (BEC) is a state of matter formed by a system of bosons confined in an external potential and cooled to temperatures very near to absolute zero (0 Kelvin or −273.15 °C). –Under such supercooled conditions, a large fraction of the atoms collapse into the lowest Quantum state of the external potential, at which point quantum effects become apparent on a macroscopic scale.
- 217. • A Bose–Einstein condensate (BEC) is a state of matter formed by a system of bosons confined in an external potential and cooled to temperatures very near to absolute zero (0 Kelvin or −273.15 °C). –Under such supercooled conditions, a large fraction of the atoms collapse into the lowest Quantum state of the external potential, at which point quantum effects become apparent on a macroscopic scale.
- 218. First predicted by Satyendra Nath Bose who wrote paper and sent to Albert Einstein
- 219. First predicted by Satyendra Nath Bose who wrote paper and sent to Albert Einstein
- 220. First predicted by Satyendra Nath Bose who wrote paper and sent to Albert Einstein
- 221. First predicted by Satyendra Nath Bose who wrote paper and sent to Albert Einstein
- 222. First predicted by Satyendra Nath Bose who wrote paper and sent to Albert Einstein
- 223. Tc = is the critical temperature, n = is the particle density, m =is the mass per boson, h = is the reduced Planck constant, Kb = is the Boltzmann constant, and is the Riemann zeta function;
- 224. Tc = is the critical temperature, n = is the particle density, m =is the mass per boson, h = is the reduced Planck constant, Kb = is the Boltzmann constant, and is the Riemann zeta function;
- 225. Copyright © 2010 Ryan P. Murphy “WHAT!”
- 226. • Plasma is super excited gas of moving electrons. Copyright © 2010 Ryan P. Murphy
- 227. • Plasma is super excited gas of moving electrons. Copyright © 2010 Ryan P. Murphy Learn More / Simplified at: http://www.colorado.edu/physics/2000/bec/what_is_it.htm l
- 228. • Bose-Einstein condensate (Optional) – http://www.youtube.com/watch?v=nAGPAb4ob s8
- 229. • Bose-Einstein condensate (Optional) – http://www.youtube.com/watch?v=nAGPAb4ob s8
- 230. • Which one is which? – Connect the name to the state of matter. • BEC Plasma Gas Liquid Solid Copyright © 2010 Ryan P. Murphy
- 231. • Which one is which? – Connect the name to the state of matter. • BEC Plasma Gas Liquid Solid Copyright © 2010 Ryan P. Murphy
- 232. • Which one is which? – Connect the name to the state of matter. • BEC Plasma Gas Liquid Solid Copyright © 2010 Ryan P. Murphy
- 233. • Which one is which? – Connect the name to the state of matter. • BEC Plasma Gas Liquid Solid Copyright © 2010 Ryan P. Murphy
- 234. • Which one is which? – Connect the name to the state of matter. • BEC Plasma Gas Liquid Solid Copyright © 2010 Ryan P. Murphy
- 235. • Which one is which? – Connect the name to the state of matter. • BEC Plasma Gas Liquid Solid Copyright © 2010 Ryan P. Murphy
- 236. • Which one is which? – Connect the name to the state of matter. • BEC Plasma Gas Liquid Solid Copyright © 2010 Ryan P. Murphy
- 237. • Which one is which? – Connect the name to the state of matter. • BEC Plasma Gas Liquid Solid Copyright © 2010 Ryan P. Murphy
- 238. • Which one is which? – Connect the name to the state of matter. • BEC Plasma Gas Liquid Solid Copyright © 2010 Ryan P. Murphy
- 239. • Which one is which? – Connect the name to the state of matter. • BEC Plasma Gas Liquid Solid Copyright © 2010 Ryan P. Murphy
- 240. • Which one is which? – Connect the name to the state of matter. • BEC Plasma Gas Liquid Solid Copyright © 2010 Ryan P. Murphy
- 241. • Video – Molecular motion of water / liquid. – Focus on how the molecules are moving as a liquid (Start) and solid (End of Video) http://www.youtube.com/watch?v=gmjLXrMaFTg Copyright © 2010 Ryan P. Murphy
- 242. Mystery box #1 Mystery Box #2 Mystery Box #3
- 243. Mystery Box #2 Mystery Box #3
- 244. Mystery Box #3
- 246. Which is a solid, which is a liquid, and which is a gas?
- 253. • Matter can exist in several different forms based on pressure, temperature and volume.
- 260. • Activity! Matter and Phase Change Simulator. – http://phet.colorado.edu/en/simulation/states-of- matter
- 261. • Video Link! (Optional) Khan Academy, • States of Matter (Advanced) – http://www.khanacademy.org/video/states-of- matter?playlist=Chemistry
- 262. • Video Short! The three states of matter. – A good review before the quiz. – http://www.youtube.com/watch?v=s- KvoVzukHo
- 263. • Activity Sheet Available: States of Matter and Phase Change.
- 264. • Quiz 1-10 Solid, Liquid, Gas, Plasma Copyright © 2010 Ryan P. Murphy
- 275. • Bonus – Name the movie and the character.
- 276. • Answers to the Quiz Wiz 1-10 States of Matter on a molecular level.
- 299. • Bonus – Name the movie and the character.
- 300. • Bonus – Name the movie and character. • Legally Blonde “Elle Woods” (2001) starring Reese Witherspoon and Luke Wilson.
- 302. • Be prepared to have more questions than answers for the next 100 slides.
- 303. • You should be on page 2 of your bundle.
- 304. • You should be on page 2 of your bundle.
- 309. • Everything from galaxies to mountains to molecules is made from small particles that make up the atom. Copyright © 2010 Ryan P. Murphy
- 310. • Everything from galaxies to mountains to molecules is made from small particles that make up the atom. – An atom has a proton, neutron, and electron. Copyright © 2010 Ryan P. Murphy
- 311. • Everything from galaxies to mountains to molecules is made from small particles that make up the atom. – An atom has a proton, neutron, and electron. – Inside the atom are quarks that make up the neutron and proton. Copyright © 2010 Ryan P. Murphy
- 312. • Everything from galaxies to mountains to molecules is made from small particles that make up the atom. – An atom has a proton, neutron, and electron. – Inside the atom are quarks that make up the neutron and proton. – Around the atom you will find the electron. Copyright © 2010 Ryan P. Murphy
- 313. • Everything from galaxies to mountains to molecules is made from small particles that make up the atom. – An atom has a proton, neutron, and electron. – Inside the atom are quarks that make up the neutron and proton. – Around the atom you will find the electron. Copyright © 2010 Ryan P. Murphy
- 314. • Everything from galaxies to mountains to molecules is made from small particles that make up the atom. – An atom has a proton, neutron, and electron. – Inside the atom are quarks that make up the neutron and proton. – Around the atom you will find the electron. Copyright © 2010 Ryan P. Murphy
- 317. • As you have just heard, everything from galaxies to mountains to molecules is made from quarks and leptons (Electrons). But that is not the whole story… – Quarks behave differently than leptons, and for each kind of matter particle there is a corresponding antimatter particle. Copyright © 2010 Ryan P. Murphy
- 318. • As you have just heard, everything from galaxies to mountains to molecules is made from quarks and leptons (Electrons). But that is not the whole story… – Quarks behave differently than leptons, and for each kind of matter particle there is a corresponding antimatter particle. Copyright © 2010 Ryan P. Murphy
- 319. • As you have just heard, everything from galaxies to mountains to molecules is made from quarks and leptons (Electrons). But that is not the whole story… – Quarks behave differently than leptons, and for each kind of matter particle there is a corresponding antimatter particle. Copyright © 2010 Ryan P. Murphy
- 320. • As you have just heard, everything from galaxies to mountains to molecules is made from quarks and leptons (Electrons). But that is not the whole story… – Quarks behave differently than leptons, and for each kind of matter particle there is a corresponding antimatter particle. Copyright © 2010 Ryan P. Murphy
- 321. • As you have just heard, everything from galaxies to mountains to molecules is made from quarks and leptons (Electrons). But that is not the whole story… – Quarks behave differently than leptons, and for each kind of matter particle there is a corresponding antimatter particle. Copyright © 2010 Ryan P. Murphy
- 322. • As you have just heard, everything from galaxies to mountains to molecules is made from quarks and leptons (Electrons). But that is not the whole story… – Quarks behave differently than leptons, and for each kind of matter particle there is a corresponding antimatter particle. Copyright © 2010 Ryan P. Murphy
- 323. • As you have just heard, everything from galaxies to mountains to molecules is made from quarks and leptons (Electrons). But that is not the whole story… – Quarks behave differently than leptons, and for each kind of matter particle there is a corresponding antimatter particle. Copyright © 2010 Ryan P. Murphy
- 324. • As you have just heard, everything from galaxies to mountains to molecules is made from quarks and leptons (Electrons). But that is not the whole story… – Quarks behave differently than leptons, and for each kind of matter particle there is a corresponding antimatter particle. Copyright © 2010 Ryan P. Murphy
- 325. • As you have just heard, everything from galaxies to mountains to molecules is made from quarks and leptons (Electrons). But that is not the whole story… – Quarks behave differently than leptons, and for each kind of matter particle there is a corresponding antimatter particle. Copyright © 2010 Ryan P. Murphy
- 326. • Scientists have observed antimatter particles for brief seconds.
- 327. • Scientists have observed antimatter particles for brief seconds. – The search is on to find out where they all are, if they are even out there?
- 328. • Scientists have observed antimatter particles for brief seconds. – The search is on to find out where they all are, if they are even out there? “Hi!”
- 329. • Scientists have observed antimatter particles for brief seconds. – The search is on to find out where they all are, if they are even out there? “Hi!”
- 330. • Scientists have observed antimatter particles for brief seconds. – The search is on to find out where they all are, if they are even out there? “Hi!” Antimatter. Learn more: http://livefromcern.web.cern.ch/livefromcern/antimatter/
- 331. • Video Link HD (Optional) The Universe, Big Bang, and Antimatter -24 minutes – Sorry for the advertisements. – http://www.youtube.com/watch?v=Y5oNwJNdMxY
- 332. • Matter – “It’s everything around us, from the air we breathe to the chair we are sitting on.” “We are made of matter.”
- 333. • Matter – “It’s everything around us, from the air we breathe to the chair we are sitting on.” “We are made of matter.”
- 334. • Matter – “It’s everything around us, from the air we breathe to the chair we are sitting on.” “We are made of matter.”
- 335. • Matter – “It’s everything around us, from the air we breathe to the chair we are sitting on.” “We are made of matter.” Copyright © 2010 Ryan P. Murphy
- 336. • “The matter that we see everyday is made up of very small particles called atoms.” Copyright © 2010 Ryan P. Murphy
- 337. • “However, Atoms only make up 5% of the Universe” “The other 95% is made of…” Copyright © 2010 Ryan P. Murphy
- 338. • “Something else.” “It is dark and…” Copyright © 2010 Ryan P. Murphy
- 339. • “Ahh…….” Copyright © 2010 Ryan P. Murphy
- 340. Copyright © 2010 Ryan P. Murphy
- 341. Copyright © 2010 Ryan P. Murphy
- 343. Dark Matter is a theory. A theory is a scientifically acceptable general principle or body of principles offered to explain phenomena. -An unproved assumption
- 344. • Most of the matter in the universe is not atoms found on planets or stars.
- 345. • Dark Matter and Dark Energy are believed to make up most of the matter in the Universe.
- 346. • Dark Matter and Dark Energy are believed to make up most of the matter in the Universe. Less than 5% of the Universe is the matter we know the most about.
- 348. “It’s called Dark Matter and Dark Energy, not because it’s evil, but because we still don’t know a lot about it.”
- 349. • Dark Matter – A hypothetical form of matter that makes up a large percentage of the universe; it’s invisible. – (Does not absorb or emit light). Copyright © 2010 Ryan P. Murphy
- 350. • Cosmic Web: A network of filaments of dark matter, believed by many astronomers to form the basis of the universe
- 364. Copyright © 2010 Ryan P. Murphy
- 365. Copyright © 2010 Ryan P. Murphy “We are still in the dark when it comes to fully understanding these forms of Matter.”
- 366. • Video Link! (Optional) Hank explains Dark Matter – http://www.youtube.com/watch?v=VL6ZNHiqP9A
- 367. • Dark Energy – A hypothetical form of energy that permeates space and exerts a negative pressure, which would have gravitational effects. – This account for the differences between the theoretical and observational results of gravitational effects on visible matter.
- 368. • Dark Energy – A hypothetical form of energy that permeates space and exerts a negative pressure, which would have gravitational effects. – This account for the differences between the theoretical and observational results of gravitational effects on visible matter.
- 369. • Video Link! (Optional) Hank explains Dark Energy. – http://www.youtube.com/watch?v=ATwVApurIQ4
- 370. • Science knows how much Dark Energy there is because we know how it affects the Universe's expansion.
- 371. • Science knows how much Dark Energy there is because we know how it affects the Universe's expansion. – Other than that, it is still mostly a mystery.
- 372. • Science knows how much Dark Energy there is because we know how it affects the Universe's expansion. – Other than that, it is still mostly a mystery.
- 373. • To describe Dark Matter in easy form for you.
- 374. • To describe Dark Matter in easy form for you. – Dark Matter
- 375. • To describe Dark Matter in easy form for you. – Dark Matter • 25% of the Universe
- 376. • To describe Dark Matter in easy form for you. – Dark Matter • 25% of the Universe • Does not absorb or emit light
- 377. • To describe Dark Matter in easy form for you. – Dark Matter • 25% of the Universe • Does not absorb or emit light • Not normal matter (Stars and Planets)
- 378. • To describe Dark Matter in easy form for you. – Dark Matter • 25% of the Universe • Does not absorb or emit light • Not normal matter (Stars and Planets) • Not anti-matter
- 379. • To describe Dark Matter in easy form for you. – Dark Matter • 25% of the Universe • Does not absorb or emit light • Not normal matter (Stars and Planets) • Not anti-matter • Possibilities for Dark Matter include MACHOs, and WIMPs.
- 380. • MACHOs • (MAssive Compact Halo Objects): Objects ranging in size from small stars to super massive black holes. MACHOS are made of ordinary matter (like protons, neutrons and electrons). They may be black holes, neutron stars, or brown dwarfs.
- 381. • MACHOs • (MAssive Compact Halo Objects): Objects ranging in size from small stars to super massive black holes. – MACHOS are made of ordinary matter (like protons, neutrons and electrons). They may be black holes, neutron stars, or brown dwarfs.
- 382. • MACHOs • (MAssive Compact Halo Objects): Objects ranging in size from small stars to super massive black holes. – MACHOS are made of ordinary matter (like protons, neutrons and electrons). They may be black holes, neutron stars, or brown dwarfs.
- 383. • WIMPs • (Weakly Interacting Massive Particles): – Subatomic particles which are not made up of ordinary matter. – They are "weakly interacting" because they can pass through ordinary matter without any effects. – They are "massive" in the sense of having mass (whether they are light or heavy depends on the particle). The prime candidates include neutrinos, axions, and neutralinos.
- 384. • WIMPs • (Weakly Interacting Massive Particles): – Subatomic particles which are not made up of ordinary matter. – They are "weakly interacting" because they can pass through ordinary matter without any effects. – They are "massive" in the sense of having mass (whether they are light or heavy depends on the particle). The prime candidates include neutrinos, axions, and neutralinos.
- 385. • WIMPs • (Weakly Interacting Massive Particles): – Subatomic particles which are not made up of ordinary matter. – They are "weakly interacting" because they can pass through ordinary matter without any effects. – They are "massive" in the sense of having mass (whether they are light or heavy depends on the particle). The prime candidates include neutrinos, axions, and neutralinos.
- 386. • WIMPs • (Weakly Interacting Massive Particles): – Subatomic particles which are not made up of ordinary matter. – They are "weakly interacting" because they can pass through ordinary matter without any effects. – They are "massive" in the sense of having mass (whether they are light or heavy depends on the particle). The prime candidates include neutrinos, axions, and neutralinos. “Oh-No”
- 387. • WIMPs • (Weakly Interacting Massive Particles): – Subatomic particles which are not made up of ordinary matter. – They are "weakly interacting" because they can pass through ordinary matter without any effects. – They are "massive" in the sense of having mass (whether they are light or heavy depends on the particle). The prime candidates include neutrinos, axions, and neutralinos.
- 388. • To describe Dark Matter and Dark Energy in easy form for you.
- 389. • To describe Dark Matter and Dark Energy in easy form for you. – Dark Matter : Helped to form Galaxies (draws matter together)
- 390. • To describe Dark Matter and Dark Energy in easy form for you. – Dark Matter : Helped to form Galaxies (draws matter together)
- 391. • To describe Dark Matter and Dark Energy in easy form for you. – Dark Matter : Helped to form Galaxies (draws matter together) – Dark Energy: A property of space? A dynamic energy field that is the opposite of normal matter and energy? A new theory of gravity? It is also pulling the universe apart (expansion).
- 392. • To describe Dark Matter and Dark Energy in easy form for you. – Dark Matter : Helped to form Galaxies (draws matter together) – Dark Energy: A property of space? A dynamic energy field that is the opposite of normal matter and energy? A new theory of gravity? It is also pulling the universe apart (expansion).
- 393. • Dark Matter – A hypothetical form of matter that is believed to make up a large percent of the universe; it is invisible (does not absorb or emit light). Copyright © 2010 Ryan P. Murphy
- 394. • Dark Matter – A hypothetical form of matter that is believed to make up a large percent of the universe; it is invisible (does not absorb or emit light). Copyright © 2010 Ryan P. Murphy Dark Matter and Dark Energy. Learn more at… http://science.nasa.gov/astrophysics/focus-areas/what- is-dark-energy/
- 395. • Video – Dark Matter • http://www.youtube.com/watch?v=nJN2X3 NrQAE
- 396. • Dark Matter explain by Michio Kaku: Video Link (Optional) • http://www.youtube.com/watch?v=e4nnpg 4N35o&feature=related
- 398. • String Theory:
- 399. • String Theory: • “The theory of everything”
- 400. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 401. • String Theory: • “The theory of everything”
- 402. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 403. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 404. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 405. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 406. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 407. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 408. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 409. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 410. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 411. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 412. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 413. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 414. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 415. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 416. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 417. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 418. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 419. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 420. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 421. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 422. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 423. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 424. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 425. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 426. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 427. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 428. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 429. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings.
- 430. • String Theory: • “The theory of everything” – A theory that sub atomic particles are actually one dimensional strings. String Theory: Learn more… http://www.nucleares.unam.mx/~alberto/physics/string.html
- 432. “Thank goodness that part is over.” “My brain hurts.”
- 434. • Activity Sheet Available: States of Matter and Phase Change.
- 435. • Mini-Experiment (Start) Copyright © 2010 Ryan P. Murphy
- 436. • Mini-Experiment (Start) – Please hold the Hershey Kiss in your hand. – Please do not squish the kiss. – Please don’t ask me if you can eat it, just hold on to it until a slide tells you to end. – Record observations in journal as a before and after drawing. Copyright © 2010 Ryan P. Murphy
- 437. • Mini-Experiment (Start) – Please hold the Hershey Kiss in your hand. – Please do not squish the kiss. – Please don’t ask me if you can eat it, just hold on to it until a slide tells you to end. – Record observations in journal as a before and after drawing. Copyright © 2010 Ryan P. Murphy
- 438. • Mini-Experiment (Start) – Please hold the Hershey Kiss in your hand. – Please do not squish the kiss. – Please don’t ask me if you can eat it, just hold on to it until a slide tells you to end. – Record observations in journal as a before and after drawing. Copyright © 2010 Ryan P. Murphy
- 439. • Mini-Experiment (Start) – Please hold the Hershey Kiss in your hand. – Please do not squish the kiss. – Please don’t ask me if you can eat it, just hold on to it until a slide tells you to end. – Record observations in journal as a before and after drawing. Copyright © 2010 Ryan P. Murphy
- 440. Physical Change Changes form solid > liquid> gas> plasma Doesn’t change identity Copyright © 2010 Ryan P. Murphy
- 441. Physical Change Changes form: solid > liquid> gas> plasma Doesn’t change identity Copyright © 2010 Ryan P. Murphy
- 442. Physical Change Changes form: solid > liquid> gas> plasma Doesn’t change identity Copyright © 2010 Ryan P. Murphy
- 443. Physical Change Changes form: solid > liquid> gas> plasma Doesn’t change identity Copyright © 2010 Ryan P. Murphy
- 444. Physical Change Changes form: solid > liquid> gas> plasma Doesn’t change identity Copyright © 2010 Ryan P. Murphy
- 445. Physical Change Changes form: solid > liquid> gas> plasma Doesn’t change identity Copyright © 2010 Ryan P. Murphy
- 446. Physical Change Changes form: solid > liquid> gas> plasma Doesn’t change identity Copyright © 2010 Ryan P. Murphy
- 447. • Physical phase change – Freezing - Liquid to a solid. – Melting - Solid to a liquid. – Sublimation- - Solid to a gas (no liquid phase) – Evaporation - Liquid to a gas. – Condensation - Gas to a liquid. Copyright © 2010 Ryan P. Murphy
- 448. • Physical phase change – Freezing - Liquid to a solid. – Melting - Solid to a liquid. – Sublimation- - Solid to a gas (no liquid phase) – Evaporation - Liquid to a gas. – Condensation - Gas to a liquid. Copyright © 2010 Ryan P. Murphy
- 449. • Physical phase change – Freezing - Liquid to a solid. – Melting - Solid to a liquid. – Sublimation- - Solid to a gas (no liquid phase) – Evaporation - Liquid to a gas. – Condensation - Gas to a liquid. Copyright © 2010 Ryan P. Murphy
- 450. • Physical phase change – Freezing - Liquid to a solid. – Melting - Solid to a liquid. – Sublimation- - Solid to a gas (no liquid phase) – Evaporation - Liquid to a gas. – Condensation - Gas to a liquid. Copyright © 2010 Ryan P. Murphy
- 451. • Physical phase change – Freezing - Liquid to a solid. – Melting - Solid to a liquid. – Sublimation- - Solid to a gas (no liquid phase) – Evaporation - Liquid to a gas. – Condensation - Gas to a liquid. Copyright © 2010 Ryan P. Murphy
- 452. • Physical phase change – Freezing - Liquid to a solid. – Melting - Solid to a liquid. – Sublimation- - Solid to a gas (no liquid phase) – Evaporation - Liquid to a gas. – Condensation - Gas to a liquid. Copyright © 2010 Ryan P. Murphy
- 453. • Physical phase change – Freezing - Liquid to a solid. – Melting - Solid to a liquid. – Sublimation- - Solid to a gas (no liquid phase) – Evaporation - Liquid to a gas. – Condensation - Gas to a liquid. Copyright © 2010 Ryan P. Murphy
- 454. • Physical phase change – Freezing - Liquid to a solid. – Melting - Solid to a liquid. – Sublimation- - Solid to a gas (no liquid phase) – Evaporation - Liquid to a gas. – Condensation - Gas to a liquid. Copyright © 2010 Ryan P. Murphy
- 455. • Physical phase change – Freezing - Liquid to a solid. – Melting - Solid to a liquid. – Sublimation- - Solid to a gas (no liquid phase) – Evaporation - Liquid to a gas. – Condensation - Gas to a liquid. Copyright © 2010 Ryan P. Murphy
- 456. • Physical phase change – Freezing - Liquid to a solid. – Melting - Solid to a liquid. – Sublimation- - Solid to a gas (no liquid phase) – Evaporation - Liquid to a gas. – Condensation - Gas to a liquid. Copyright © 2010 Ryan P. Murphy
- 457. • Physical phase change – Freezing - Liquid to a solid. – Melting - Solid to a liquid. – Sublimation- - Solid to a gas (no liquid phase) – Evaporation - Liquid to a gas. – Condensation - Gas to a liquid. Copyright © 2010 Ryan P. Murphy
- 458. • Physical phase change – Freezing - Liquid to a solid. – Melting - Solid to a liquid. – Sublimation- - Solid to a gas (no liquid phase) – Evaporation - Liquid to a gas. – Condensation - Gas to a liquid. Copyright © 2010 Ryan P. Murphy
- 459. • Physical phase change – Freezing - Liquid to a solid. – Melting - Solid to a liquid. – Sublimation- - Solid to a gas (no liquid phase) – Evaporation - Liquid to a gas. – Condensation - Gas to a liquid. Copyright © 2010 Ryan P. Murphy
- 460. • Physical phase change – Freezing - Liquid to a solid. – Melting - Solid to a liquid. – Sublimation- - Solid to a gas (no liquid phase) – Evaporation - Liquid to a gas. – Condensation - Gas to a liquid. Copyright © 2010 Ryan P. Murphy
- 461. • Physical phase change – Freezing - Liquid to a solid. – Melting - Solid to a liquid. – Sublimation- - Solid to a gas (no liquid phase) – Evaporation - Liquid to a gas. – Condensation - Gas to a liquid. Copyright © 2010 Ryan P. Murphy
- 462. • Physical phase change – Freezing - Liquid to a solid. – Melting - Solid to a liquid. – Sublimation- - Solid to a gas (no liquid phase) – Evaporation - Liquid to a gas. – Condensation - Gas to a liquid. Copyright © 2010 Ryan P. Murphy
- 463. • Physical phase change – Freezing - Liquid to a solid. – Melting - Solid to a liquid. – Sublimation- - Solid to a gas (no liquid phase) – Evaporation - Liquid to a gas. – Condensation - Gas to a liquid. Copyright © 2010 Ryan P. Murphy
- 464. • Physical phase change – Freezing - Liquid to a solid. – Melting - Solid to a liquid. – Sublimation- - Solid to a gas (no liquid phase) – Evaporation - Liquid to a gas. – Condensation - Gas to a liquid. Copyright © 2010 Ryan P. Murphy
- 465. • Physical phase change – Freezing - Liquid to a solid. – Melting - Solid to a liquid. – Sublimation- - Solid to a gas (no liquid phase) – Evaporation - Liquid to a gas. – Condensation - Gas to a liquid. Copyright © 2010 Ryan P. Murphy
- 466. • Physical phase change – Freezing - Liquid to a solid. – Melting - Solid to a liquid. – Sublimation- - Solid to a gas (no liquid phase) – Evaporation - Liquid to a gas. – Condensation - Gas to a liquid. Copyright © 2010 Ryan P. Murphy
- 467. • Please sketch the following into your science journal (Half Page needed). Copyright © 2010 Ryan P. Murphy
- 468. Copyright © 2010 Ryan P. Murphy
- 469. Copyright © 2010 Ryan P. Murphy
- 470. Copyright © 2010 Ryan P. Murphy
- 471. Copyright © 2010 Ryan P. Murphy
- 472. Copyright © 2010 Ryan P. Murphy
- 473. Copyright © 2010 Ryan P. Murphy
- 474. Copyright © 2010 Ryan P. Murphy
- 475. Copyright © 2010 Ryan P. Murphy
- 476. Copyright © 2010 Ryan P. Murphy
- 477. Copyright © 2010 Ryan P. Murphy
- 478. Copyright © 2010 Ryan P. Murphy
- 479. Copyright © 2010 Ryan P. Murphy
- 481. Copyright © 2010 Ryan P. Murphy
- 482. Copyright © 2010 Ryan P. Murphy
- 483. Copyright © 2010 Ryan P. Murphy
- 484. Copyright © 2010 Ryan P. Murphy
- 485. Copyright © 2010 Ryan P. Murphy
- 486. Copyright © 2010 Ryan P. Murphy
- 487. Copyright © 2010 Ryan P. Murphy
- 488. Copyright © 2010 Ryan P. Murphy
- 489. Copyright © 2010 Ryan P. Murphy
- 490. • Activity Sheet Available: States of Matter and Phase Change.
- 491. • Mini-Experiment (End) – What happened to the kiss? – Draw the after picture. – What was added to cause the phase change? • Describe this next to your after drawing. Copyright © 2010 Ryan P. Murphy
- 492. • Mini-Experiment (End) – What happened to the kiss? – Draw the after picture. – What was added to cause the phase change? • Describe this next to your after drawing. Copyright © 2010 Ryan P. Murphy
- 493. • Mini-Experiment (End) – What happened to the kiss? – Draw the after picture. – What was added to cause the phase change? • Describe this next to your after drawing. Copyright © 2010 Ryan P. Murphy
- 494. • Mini-Experiment (End) – What happened to the kiss? – Draw the after picture. – What was added to cause the phase change? • Describe this next to your after drawing. Copyright © 2010 Ryan P. Murphy
- 495. • A physical change / reaction can also occur with with nucleation sites. Copyright © 2010 Ryan P. Murphy
- 496. • Nucleation site: A place that acts as a nucleus for (starting), in a process of formation such as crystals, or bubbles. Copyright © 2010 Ryan P. Murphy
- 497. • Activity! I’m going to give you a tablet with many nucleation sites on it. Drop into the aqueous solution and record observations. Copyright © 2010 Ryan P. Murphy
- 498. • Diet Coke and Mentos is a physical reaction not a chemical reaction. –Occurs because of nucleation sites. Copyright © 2010 Ryan P. Murphy
- 499. • Video: Nucleation Sites. • http://www.youtube.com/watch?v=9vk4_2x boOE Copyright © 2010 Ryan P. Murphy
- 500. • Acetone and Styrofoam: The difference between melting and dissolving. – Safety Goggles and Gloves Required. – Learn more at… • http://www.elmhurst.edu/~chm/demos/Disappearing Cup.html
- 501. • Demonstration: What happened to the cup when placed on water?
- 502. • Demonstration: What happened to the cup when placed on water? “Dude, that was wicked boring.”
- 503. • Demonstration: Melting vs. Dissolving – http://www.youtube.com/watch?v=h9Jx8NRkWTo • Precautions: Uses Acetone, requires safety goggles, ventilated area, and acetone is flammable.
- 504. • Dissolving: To become incorporated into a liquid so as to form a solution. • Melting: To be changed from a solid to a liquid state especially by the application of heat.
- 505. • Dissolving: To become incorporated into a liquid so as to form a solution. • Melting: To be changed from a solid to a liquid state especially by the application of heat.
- 506. • Dissolving: To become incorporated into a liquid so as to form a solution. • Melting: To be changed from a solid to a liquid state especially by the application of heat.
- 507. • Dissolving: To become incorporated into a liquid so as to form a solution. • Melting: To be changed from a solid to a liquid state especially by the application of heat.
- 508. • Dissolving: To become incorporated into a liquid so as to form a solution. • Melting: To be changed from a solid to a liquid state especially by the application of heat.
- 509. • Dissolving: To become incorporated into a liquid so as to form a solution. • Melting: To be changed from a solid to a liquid state especially by the application of heat.
- 510. • Dissolving: To become incorporated into a liquid so as to form a solution. • Melting: To be changed from a solid to a liquid state especially by the application of heat.
- 511. • Demonstration: Melting vs. Dissolving • Who wants to read the answer aloud to the class? • When the acetone was poured into the cup, the cup dissolved. (Avoid saying that the cup melts, because this is not true). The reason for this happening is because the acetone and the Styrofoam cup share the same properties, they are both non- polar. Likes dissolves likes. Non- polar things have no charge, and polar things have positive and negative charges. The Styrofoam cup didn't dissolve with the water because, they have different properties, the water is polar, and the cup is non-polar. Acetone is actually what girls use to take off their nail polish.
- 512. • Demonstration: Melting vs. Dissolving • Who wants to read the answer aloud to the class? • When the acetone was poured into the cup, the cup dissolved. (Avoid saying that the cup melts, because this is not true). The reason for this happening is because the acetone and the Styrofoam cup share the same properties, they are both non- polar. Likes dissolves likes. Non- polar things have no charge, and polar things have positive and negative charges. The Styrofoam cup didn't dissolve with the water because, they have different properties, the water is polar, and the cup is non-polar. Acetone is actually what girls use to take off their nail polish.
- 513. • Demonstration: Melting vs. Dissolving • Who wants to read the answer aloud to the class? • When the acetone was poured into the cup, the cup dissolved. (Avoid saying that the cup melts, because this is not true). The reason for this happening is because the acetone and the Styrofoam cup share the same properties, they are both non- polar. Likes dissolves likes. Non- polar things have no charge, and polar things have positive and negative charges. The Styrofoam cup didn't dissolve with the water because, they have different properties, the water is polar, and the cup is non-polar. Acetone is actually what girls use to take off their nail polish.
- 514. • Demonstration: Melting vs. Dissolving • Who wants to read the answer aloud to the class? • When the acetone was poured into the cup, the cup dissolved. (Avoid saying that the cup melts, because this is not true). The reason for this happening is because the acetone and the Styrofoam cup share the same properties, they are both non- polar. Likes dissolves likes. Non- polar things have no charge, and polar things have positive and negative charges. The Styrofoam cup didn't dissolve with the water because, they have different properties, the water is polar, and the cup is non-polar. Acetone is actually what girls use to take off their nail polish.
- 515. • Demonstration: Melting vs. Dissolving • Who wants to read the answer aloud to the class? • When the acetone was poured into the cup, the cup dissolved. (Avoid saying that the cup melts, because this is not true). The reason for this happening is because the acetone and the Styrofoam cup share the same properties, they are both non- polar. Likes dissolves likes. Non- polar things have no charge, and polar things have positive and negative charges. The Styrofoam cup didn't dissolve with the water because, they have different properties, the water is polar, and the cup is non-polar. Acetone is actually what girls use to take off their nail polish.
- 516. • Demonstration: Melting vs. Dissolving • Who wants to read the answer aloud to the class? • When the acetone was poured into the cup, the cup dissolved. (Avoid saying that the cup melts, because this is not true). The reason for this happening is because the acetone and the Styrofoam cup share the same properties, they are both non- polar. Likes dissolves likes. Non- polar things have no charge, and polar things have positive and negative charges. The Styrofoam cup didn't dissolve with the water because, they have different properties, the water is polar, and the cup is non-polar. Acetone is actually what girls use to take off their nail polish.
- 517. • Demonstration: Melting vs. Dissolving • Who wants to read the answer aloud to the class? • When the acetone was poured into the cup, the cup dissolved. (Avoid saying that the cup melts, because this is not true). The reason for this happening is because the acetone and the Styrofoam cup share the same properties, they are both non- polar. Likes dissolves likes. Non- polar things have no charge, and polar things have positive and negative charges. The Styrofoam cup didn't dissolve with the water because, they have different properties, the water is polar, and the cup is non-polar. Acetone is actually what girls use to take off their nail polish.
- 518. • Demonstration: Melting vs. Dissolving • Who wants to read the answer aloud to the class? • When the acetone was poured into the cup, the cup dissolved. (Avoid saying that the cup melts, because this is not true). The reason for this happening is because the acetone and the Styrofoam cup share the same properties, they are both non- polar. Likes dissolves likes. Non- polar things have no charge, and polar things have positive and negative charges. The Styrofoam cup didn't dissolve with the water because they have different properties. the water is polar, and the cup is non-polar. Acetone is actually what girls use to take off their nail polish.
- 519. • Demonstration: Melting vs. Dissolving • Who wants to read the answer aloud to the class? • When the acetone was poured into the cup, the cup dissolved. (Avoid saying that the cup melts, because this is not true). The reason for this happening is because the acetone and the Styrofoam cup share the same properties, they are both non- polar. Likes dissolves likes. Non- polar things have no charge, and polar things have positive and negative charges. The Styrofoam cup didn't dissolve with the water because they have different properties. The water is polar, and the cup is non-polar. Acetone is actually what girls use to take off their nail polish.
- 520. • Activity – Temperature and phase change – Using digital thermometers and water. – Record temperature of water every minute. – Record observations of phase change during the minute they occur. • Bubbles – Boiling starts • Gas release – Boiling Copyright © 2010 Ryan P. Murphy
- 521. • Activity Sheet Available: States of Matter and Phase Change. – State of Matter Lab
- 522. • Please sketch the following graph in your journal. 100 90 80 70 60 50 40 30 20 10 0 -10 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 D E G R E E S C E L S I U S
- 523. • Please sketch the following graph in your journal. 100 90 80 70 60 50 40 30 20 10 0 -10 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Degrees Celsius
- 524. • Please sketch the following graph in your journal. 100 90 80 70 60 50 40 30 20 10 0 -10 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Degrees Celsius Time / Minutes
- 525. • Please sketch the following graph in your journal. 100 90 80 70 60 50 40 30 20 10 0 -10 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Degrees Celsius Time / Minutes
- 526. • Please sketch the following graph in your journal. 100 90 80 70 60 50 40 30 20 10 0 -10 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
- 527. • Activity – Temperature and phase change – Using digital thermometers and water. – Record temperature of ice water every minute. Tape thermometer so it doesn’t touch the side of the container. – Record observations of phase change during the minute they occur. • Bubbles – Boiling starts • Gas release – Boiling Copyright © 2010 Ryan P. Murphy
- 528. • Activity Sheet Available: States of Matter and Phase Change.
- 529. • Questions / Analysis of graph. – Please label the states of matter as they occur on your graph (based on observations and use correct terms such as melting and vaporization). – What happened at 100 degrees Celsius? – What trends to you see in the data? Copyright © 2010 Ryan P. Murphy
- 530. • Questions / Analysis of graph. – Please label the states of matter as they occur on your graph (based on observations and use correct terms such as melting and vaporization). – What happened at 100 degrees Celsius? – What trends to you see in the data? Copyright © 2010 Ryan P. Murphy
- 531. • Questions / Analysis of graph. – Please label the states of matter as they occur on your graph (based on observations and use correct terms such as melting and vaporization). – What happened at 100 degrees Celsius? – What trends to you see in the data? Copyright © 2010 Ryan P. Murphy
- 532. • Questions / Analysis of graph. – Please label the states of matter as they occur on your graph (based on observations and use correct terms such as melting and vaporization). – What happened at 100 degrees Celsius? – What trends to you see in the data? Copyright © 2010 Ryan P. Murphy
- 533. • Questions / Analysis of graph. – Please label the states of matter as they occur on your graph (based on observations and use correct terms such as melting and vaporization). Copyright © 2010 Ryan P. Murphy
- 534. • Possible answer based on the energy added to the water. 100 90 80 70 60 50 40 30 20 10 0 -10 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
- 535. • Possible answer based on the energy added to the water. 100 90 80 70 60 50 40 30 20 10 0 -10 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Water and Ice
- 536. • Possible answer based on the energy added to the water. 100 90 80 70 60 50 40 30 20 10 0 -10 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Water and Ice Liquid
- 537. • Possible answer based on the energy added to the water. 100 90 80 70 60 50 40 30 20 10 0 -10 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Water and Ice Liquid Bubbles / Vapor
- 538. • Possible answer based on the energy added to the water. 100 90 80 70 60 50 40 30 20 10 0 -10 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Water and Ice Liquid Bubbles / VaporBoiling
- 539. • Answer to the activity. – Did your graph look the same as this? Heat added Temperature(oC) 0 100 Melting Ice Water Water Vapor Boiling Copyright © 2010 Ryan P. Murphy
- 540. • Questions / Analysis of graph. – What happened at 100 degrees Celsius? Copyright © 2010 Ryan P. Murphy
- 541. • Questions / Analysis of graph. – What happened at 100 degrees Celsius? – The water began to boil turning from a liquid to a gas. Copyright © 2010 Ryan P. Murphy
- 542. • Questions / Analysis of graph. – What trends to you see in the data? Copyright © 2010 Ryan P. Murphy
- 543. • Questions / Analysis of graph. – What trends to you see in the data? – The graph looks like a staircase. There are periods where the temperature does not change. Copyright © 2010 Ryan P. Murphy
- 544. When energy is added – Move up a step. When energy is removed – Go down a step. Heat added Temperature(oC) 0 100 Melting Ice Water Water Vapor Boiling Copyright © 2010 Ryan P. Murphy
- 545. When energy is added – Move up a step. When energy is removed – Go down a step. Heat added Temperature(oC) 0 100 Melting Ice Water Water Vapor Boiling Copyright © 2010 Ryan P. Murphy
- 546. When energy is added – Move up a step. When energy is removed – Go down a step. Heat added Temperature(oC) 0 100 Melting Ice Water Water Vapor Boiling Copyright © 2010 Ryan P. Murphy
- 547. When energy is added – Move up a step. When energy is removed – Go down a step. Heat added Temperature(oC) 0 100 Melting Ice Water Water Vapor Boiling Copyright © 2010 Ryan P. Murphy
- 548. When energy is added – Move up a step. When energy is removed – Go down a step. Heat added Temperature(oC) 0 100 Melting Ice Water Water Vapor Boiling Copyright © 2010 Ryan P. Murphy
- 549. • You may see this diagram on a state test. – When energy is added – move up a step. – When energy is removed – go down a step. Copyright © 2010 Ryan P. Murphy
- 550. –That's why water doesn't get hotter as it is boiling. The temperature remains constant until the phase change is complete. Copyright © 2010 Ryan P. Murphy
- 551. –That's why water doesn't get hotter as it is boiling. The temperature remains constant until the phase change is complete. Copyright © 2010 Ryan P. Murphy
- 552. • However, during the phase change, the temperature stays the same even though the heat energy changes. –That's why water doesn't get hotter as it is boiling. The temperature remains constant until the phase change is complete. Copyright © 2010 Ryan P. Murphy
- 553. • However, during the phase change, the temperature stays the same even though the heat energy changes. –This energy is going into changing the phase and not into raising the temperature. That's why water doesn't get hotter as it is boiling. The temperature remains constant until the phase change is complete. Copyright © 2010 Ryan P. Murphy
- 554. • However, during the phase change, the temperature stays the same even though the heat energy changes. –This energy is going into changing the phase and not into raising the temperature. That's why water doesn't get hotter as it’s boiling. The temperature remains constant until the phase change is complete. Copyright © 2010 Ryan P. Murphy
- 555. • However, during the phase change, the temperature stays the same even though the heat energy changes. –This energy is going into changing the phase and not into raising the temperature. That's why water doesn't get hotter as it’s boiling. The temperature remains constant until the phase change is complete. Copyright © 2010 Ryan P. Murphy
- 556. Latent Heat: The energy absorbed or released when a substance changes its physical state. Heat added Temperature(oC) 0 100 MeltingIce Water Water VaporBoiling Latent Heat Latent Heat Copyright © 2010 Ryan P. Murphy
- 557. Latent Heat: The energy absorbed or released when a substance changes its physical state. Heat added Temperature(oC) 0 100 MeltingIce Water Water VaporBoiling Latent Heat Latent Heat Copyright © 2010 Ryan P. Murphy
