Cartesian Diver, Gas Laws, Build a Cartesian Diver Physical Science Lesson PowerPoint
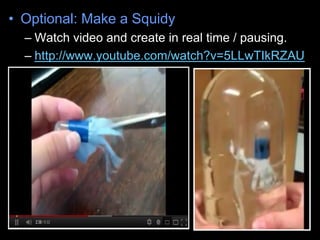
- 1. • Optional: Make a Squidy – Watch video and create in real time / pausing. – http://www.youtube.com/watch?v=5LLwTIkRZAU
- 3. • RED SLIDE: These are notes that are very important and should be recorded in your science journal. Copyright © 2010 Ryan P. Murphy
- 4. -Nice neat notes that are legible and use indentations when appropriate. -Example of indent. -Skip a line between topics -Don’t skip pages -Make visuals clear and well drawn. Please label. Ice Melting Water Boiling Vapor GasT E M P Heat Added
- 5. • RED SLIDE: These are notes that are very important and should be recorded in your science journal. • BLACK SLIDE: Pay attention, follow directions, complete projects as described and answer required questions neatly. Copyright © 2010 Ryan P. Murphy
- 7. New Area of Focus: Gases and Other Laws. Copyright © 2010 Ryan P. Murphy
- 8. Charles Law: Volume of a gas increases with temperature. (Gases expand with heat). Copyright © 2010 Ryan P. Murphy
- 10. The formula for the law is: Volume ________ = K Temp Copyright © 2010 Ryan P. Murphy
- 11. The formula for the law is: Volume ________ = K Temp Copyright © 2010 Ryan P. Murphy
- 12. Copyright © 2010 Ryan P. Murphy
- 13. V is the volume of the gas. Copyright © 2010 Ryan P. Murphy
- 14. V is the volume of the gas. T is the temperature of the gas (measured in Kelvin) Copyright © 2010 Ryan P. Murphy
- 15. V is the volume of the gas. T is the temperature of the gas (measured in Kelvin) K is a constant. Copyright © 2010 Ryan P. Murphy
- 16. V is the volume of the gas. T is the temperature of the gas (measured in Kelvin) K is a constant. K= The universal constant in the gas equation: pressure times volume = R times temperature; equal to 8.3143 joules per Kelvin per mole. Copyright © 2010 Ryan P. Murphy
- 17. V is the volume of the gas. T is the temperature of the gas (measured in Kelvin) K is a constant. K= The universal constant in the gas equation: pressure times volume = R times temperature; equal to 8.3143 joules per Kelvin per mole. Copyright © 2010 Ryan P. Murphy
- 18. V is the volume of the gas. T is the temperature of the gas (measured in Kelvin) K is a constant. K= The universal constant in the gas equation: pressure times volume = R times temperature; equal to 8.3143 joules per Kelvin per mole. Copyright © 2010 Ryan P. Murphy
- 19. • Demonstration: Fit a balloon to the top of a glass bottle and place in pan with water. – Place on top of heat source and observe.
- 20. • Demonstration: Fit a balloon to the top of a glass bottle and place in pan with water. – Place on top of heat source and observe.
- 21. • This law means that when the temperature goes up, the volume of the gas goes up.
- 22. • This law means that when the temperature goes up, the volume of the gas goes up.
- 23. • This law means that when the temperature goes up, the volume of the gas goes up.
- 24. • This law means that when the temperature goes up, the volume of the gas goes up.
- 25. • This law means that when the temperature goes up, the volume of the gas goes up.
- 26. • This law means that when the temperature goes up, the volume of the gas goes up.
- 27. • This law means that when the temperature goes up, the volume of the gas goes up.
- 28. • This law means that when the temperature goes up, the volume of the gas goes up.
- 29. • This law means that when the temperature goes up, the volume of the gas goes up.
- 30. • This law means that when the temperature goes up, the volume of the gas goes up.
- 31. • This law means that when the temperature goes up, the volume of the gas goes up.
- 32. • This law means that when the temperature goes up, the volume of the gas goes up.
- 33. • This law means that when the temperature goes up, the volume of the gas goes up. When the temperature goes down, the volume of the gas decreases.
- 34. • Set up of Demonstration. – . Copyright © 2010 Ryan P. Murphy
- 35. • Set up of Demonstration. – Blow up two similar balloons so they have the same circumference. – Place balloon in ice water on one side to 500 ml. – Place equal balloon in hot water on one side to 500ml. – Put small block and weight, or use finger to depress balloon under water. – Record difference in volume between the balloons. Copyright © 2010 Ryan P. Murphy
- 36. • Set up of Demonstration. – Blow up two similar balloons so they have the same circumference. – Place balloon in ice water on one side to 500 ml. – Place equal balloon in hot water on one side to 500ml. – Put small block and weight, or use finger to depress balloon under water. – Record difference in volume between the balloons. Copyright © 2010 Ryan P. Murphy
- 37. • Set up of Demonstration. – Blow up two similar balloons so they have the same circumference. – Place balloon in ice water on one side to 500 ml. – Place equal balloon in hot water on one side to 500ml. – Put small block and weight, or use finger to depress balloon under water. – Record difference in volume between the balloons. Copyright © 2010 Ryan P. Murphy
- 38. • Set up of Demonstration. – Blow up two similar balloons so they have the same circumference. – Place balloon in ice water on one side to 500 ml. – Place equal balloon in hot water on one side to 500ml. – Put small block and weight, or use finger to depress balloon under water. – Record difference in volume between the balloons. Copyright © 2010 Ryan P. Murphy
- 39. • Set up of Demonstration. – Blow up two similar balloons so they have the same circumference. – Place balloon in ice water on one side to 500 ml. – Place equal balloon in hot water on one side to 500ml. – Put small block and weight, or use finger to depress balloon under water. – Record difference in volume between the balloons. Copyright © 2010 Ryan P. Murphy
- 40. • Using Charles law, what will happen to the two balloons below? Copyright © 2010 Ryan P. Murphy
- 41. • Set up of demonstration. Copyright © 2010 Ryan P. Murphy
- 42. • Questions to demonstration. – Sketch the difference between the two. – How does temperature effect the volume of a gas? Think about the gas molecules in each balloon. • Use observations to back up your answers. Copyright © 2010 Ryan P. Murphy
- 43. • When temperatures get colder, you may need to add some more molecules to get the safe PSI for your vehicle. Copyright © 2010 Ryan P. Murphy
- 44. • You may notice that your sports equipment doesn’t work well when you go out into your garage in the winter. –The air molecules are moving very slowly so the ball is flat. Copyright © 2010 Ryan P. Murphy
- 45. • You may notice that your sports equipment doesn’t work well when you go out into your garage in the winter. Copyright © 2010 Ryan P. Murphy
- 47. • Avogadro’s Law / Hypothesis. Copyright © 2010 Ryan P. Murphy
- 48. • Avogadro’s Law / Hypothesis. “Hello ladies, I am the Italian savant named Amedo Avogadro.” Copyright © 2010 Ryan P. Murphy
- 49. • Avogadro’s Law / Hypothesis. “Hello ladies, I am the Italian savant named Amedo Avogadro.” – “I would love to show you my gas laws, will you join me?” Copyright © 2010 Ryan P. Murphy
- 50. • Avogadro’s Law / Hypothesis. “Hello ladies, I am the Italian savant named Amedo Avogadro.” – “I would love to show you my gas laws, will you join me?” Copyright © 2010 Ryan P. Murphy
- 51. Avogadro's Law: Equal volumes of gases, at the same temperature and pressure, contain the same number of particles, or molecules. Copyright © 2010 Ryan P. Murphy
- 52. • Gas Laws and more available sheet.
- 53. • Gas Laws and more available sheet.
- 54. • Activity! Pressure and Volume Copyright © 2010 Ryan P. Murphy
- 55. • Activity! Pressure and Volume Copyright © 2010 Ryan P. Murphy Do not over pump the “Fizz Keeper” or it can shoot-off violently. Please wear safety goggles!
- 56. • Activity! Pressure and Volume – Drop a small tied balloon into a plastic soda bottle. Copyright © 2010 Ryan P. Murphy
- 57. • Activity! Pressure and Volume – Drop a small tied balloon into a plastic soda bottle. – Cap bottle with the “Fizz Keeper” and pump many times. Copyright © 2010 Ryan P. Murphy
- 58. • Activity! Pressure and Volume – Drop a small tied balloon into a plastic soda bottle. – Cap bottle with the “Fizz Keeper” and pump many times. – Observe what happens to the balloon during the pressurizing. Copyright © 2010 Ryan P. Murphy
- 59. • Activity! Pressure and Volume – Drop a small tied balloon into a plastic soda bottle. – Cap bottle with the “Fizz Keeper” and pump many times. – Observe what happens to the balloon during the pressurizing. – Unscrew cap and observe balloon. Copyright © 2010 Ryan P. Murphy
- 60. • Balloon and Fizz Keeper Questions. – What happened to the balloon when pressure was added and then removed? – What is the connection between pressure and volume of a gas? Copyright © 2010 Ryan P. Murphy
- 61. • Balloon and Fizz Keeper Questions. – What happened to the balloon when pressure was added and then removed? Copyright © 2010 Ryan P. Murphy
- 62. • Balloon and Fizz Keeper Questions. – What happened to the balloon when pressure was added and then removed? – Answer: The balloon got smaller when the pressure was added and then larger when removed. Copyright © 2010 Ryan P. Murphy
- 63. • Balloon and Fizz Keeper Questions. – What is the connection between pressure and volume of a gas? Copyright © 2010 Ryan P. Murphy
- 64. • Balloon and Fizz Keeper Questions. – What is the connection between pressure and volume of a gas? – Answer: When pressure was increased, volume of the gas decreased. When pressure was decreased, volume increased. Copyright © 2010 Ryan P. Murphy
- 65. • Which container below has the lowest air pressure if the balloons are similar? Copyright © 2010 Ryan P. Murphy
- 66. • Which container below has the lowest air pressure if the balloons are similar? Copyright © 2010 Ryan P. Murphy
- 67. • Answer: The one on the right because the balloon has expanded since it has less pressure acting on it. Copyright © 2010 Ryan P. Murphy
- 68. • The container on the left must have higher air pressure because it is decreasing the volume of the gas in the balloon. Copyright © 2010 Ryan P. Murphy
- 69. Boyle’s Law: Pressure and Volume are inversely proportional. Copyright © 2010 Ryan P. Murphy
- 70. As pressure increases, volume decreases. As volume decreases, pressure increases. Copyright © 2010 Ryan P. Murphy
- 71. As pressure increases, volume decreases. As volume decreases, pressure increases. Copyright © 2010 Ryan P. Murphy
- 72. As pressure increases, volume decreases. As volume decreases, pressure increases. Copyright © 2010 Ryan P. Murphy
- 73. As pressure increases, volume decreases. As volume decreases, pressure increases. Copyright © 2010 Ryan P. Murphy
- 74. As pressure increases, volume decreases. As volume decreases, pressure increases. Copyright © 2010 Ryan P. Murphy “I’m Pressure.”
- 75. As pressure increases, volume decreases. As volume decreases, pressure increases. Copyright © 2010 Ryan P. Murphy “I’m Pressure.” “I’m Volume.”
- 77. Very Important! Record in Journal.
- 78. • Gas Laws and more available sheet.
- 80. • Activity! Syringes (Safety Goggles Needed)
- 81. • Activity! Syringes – Depress plunger on the syringe.
- 82. • Activity! Syringes – Depress plunger on the syringe. – Cover hole with finger.
- 83. • Activity! Syringes – Depress plunger on the syringe. – Cover hole with finger. – Try and pull handle (gently please). • Why is it difficult? Keep thumb on opening.
- 84. • Activity! Syringes – Depress plunger on the syringe. – Cover hole with finger. – Try and pull handle (gently please). • Why is it difficult? Keep thumb on opening.
- 85. • Activity! Syringes – Answer: It was difficult because your finger created a sealed vacuum and prevented air from entering the chamber. Keep thumb on opening.
- 86. • Activity! Syringes – Answer: It was difficult because your finger created a sealed vacuum and prevented air from entering the chamber. Atmospheric pressure is 1 kilogram per square centimeter at sea level. Keep thumb on opening.
- 87. • Gas Laws and more available sheet.
- 88. • Activity! Syringes (Opposite)
- 89. • Activity! Syringes (Opposite) – Fill syringe.
- 90. • Activity! Syringes (Opposite) – Fill syringe. – Cover hole with finger.
- 91. • Activity! Syringes (Opposite) – Fill syringe. – Cover hole with finger. – Try and push handle (gently please).
- 92. • Activity! Syringes (Opposite) – Fill syringe. – Cover hole with finger. – Try and push handle (gently please). • How does this represent Boyles Law?
- 93. • Activity! Syringes (Opposite) • How does this represent Boyles Law?
- 94. • Activity! Syringes (Opposite) • How does this represent Boyles Law? • Answer: As you depress the plunger, you increase pressure and the volume of the gas is decreased.
- 95. • Activity! Syringes (Opposite) • How does this represent Boyles Law? • Answer: As you depress the plunger, you increase pressure and the volume of the gas is decreased. • Please determine how many milliliters you were able to compress the gas inside using the numbers on the syringe.
- 96. • Activity! Syringes (Opposite) • How does this represent Boyles Law? • Answer: As you depress the plunger, you increase pressure and the volume of the gas is decreased. • Please determine how many milliliters you were able to compress the gas inside using the numbers on the syringe. • Answer: You should be able to compress the gas to about 50% of it’s starting volume by hand and then it gets difficult.
- 99. “Can’t wait to eat my yogurt.”
- 101. • As you inhale, your diaphragm flattens out allowing your chest to expand and allows more air to flow into your lungs.
- 102. • As you inhale, your diaphragm flattens out allowing your chest to expand and allows more air to flow into your lungs. – Air pressure decrease, air then rushes into your lungs.
- 103. • As you exhale, your diaphragm relaxes to a normal state. Space in chest decreases.
- 104. • As you exhale, your diaphragm relaxes to a normal state. Space in chest decreases. – Air pressure increases, air then rushes out of your lungs.
- 105. • Which is a inhale, and which is a exhale? A B
- 106. • Which is a inhale, and which is a exhale? • A B
- 107. • Which is a inhale, and which is a exhale? • Inhale A B
- 108. • Which is a inhale, and which is a exhale? • Inhale A B
- 109. • Which is a inhale, and which is a exhale? • Inhale Exhale A B
- 110. • Which is a inhale, and which is a exhale? A BA B
- 111. • Which is a inhale, and which is a exhale? A BA B
- 112. • Which is a inhale, and which is a exhale? • Inhale A BA B
- 113. • Which is a inhale, and which is a exhale? • Inhale A BA B
- 114. • Which is a inhale, and which is a exhale? • Inhale Exhale A BA B
- 117. • The Bends (Decompression Sickness) – Bubbles form in blood if you rise to quickly because of the rapid decrease in pressure. Copyright © 2010 Ryan P. Murphy
- 118. • The Bends (Decompression Sickness) – Bubbles form in blood if you rise to quickly because of the rapid decrease in pressure. – A diver must save time to travel to surface slowly so body can adjust. Copyright © 2010 Ryan P. Murphy
- 119. • Short Area of Focus: Last bit about air pressure (Flight). Copyright © 2010 Ryan P. Murphy
- 121. • How do planes fly?
- 122. • Early plane (Wright Brothers)
- 126. • Flight: A Simple combination of Bernoulli’s Principle and Newtons 1st Law of Motion. Copyright © 2010 Ryan P. Murphy
- 128. • An object at rest tends to stay at rest and an object in motion tends to stay in motion with the same speed and in the same direction unless acted upon by an unbalanced force. Copyright © 2010 Ryan P. Murphy
- 130. Bernoulli's Principle Bernoulli's principle states that for an inviscid flow, an increase in the speed of the fluid occurs simultaneously with a decrease in pressure or a decrease in the fluid's potential energy.
- 131. • Air flows faster over the top of the wing than the bottom making less pressure, higher pressure underneath pushes the wing up. Copyright © 2010 Ryan P. Murphy
- 132. Learn more about flight at… http://www.lcse.umn.edu/~bruff/ber noulli.html
- 134. • Propeller uses same principles with air pressure. Copyright © 2010 Ryan P. Murphy
- 135. • Activity! Teacher will demonstrate ping pong ball levitation with hair dryer. – The airflow from the hair dryer speeds up as it slips by the floating sphere, which creates an area of low pressure around the ball. The high pressure from the dryer surrounds the low around the ball and keeps the ball trapped in midair.
- 136. • Activity! Teacher will demonstrate ping pong ball levitation with hair dryer. – The airflow from the hair dryer speeds up as it slips by the floating sphere, which creates an area of low pressure around the ball. The high pressure from the dryer surrounds the low around the ball and keeps the ball trapped in midair.
- 137. • Activity! Teacher will demonstrate ping pong ball levitation with hair dryer. – The airflow from the hair dryer speeds up as it slips by the floating sphere, which creates an area of low pressure around the ball. The high pressure from the dryer surrounds the low around the ball and keeps the ball trapped in midair.
- 138. • Activity! Teacher will demonstrate ping pong ball levitation with hair dryer. – The airflow from the hair dryer speeds up as it slips by the floating sphere, which creates an area of low pressure around the ball. The high pressure from the dryer surrounds the low around the ball and keeps the ball trapped in midair.
- 139. • Activity! Teacher will demonstrate ping pong ball levitation with hair dryer. – The airflow from the hair dryer speeds up as it slips by the floating sphere, which creates an area of low pressure around the ball. The high pressure from the dryer surrounds the low around the ball and keeps the ball trapped in midair.
- 140. • Activity! – Everyone can try with a bendy straw and ping pong ball.
- 141. • Activity! – Everyone can try with a bendy straw and ping pong ball.
- 142. • Activity! – Everyone can try with a bendy straw and ping pong ball.
- 143. • Activity! – Everyone can try with a bendy straw and ping pong ball. The stream of air moves at high speed.
- 144. • Activity! – Everyone can try with a bendy straw and ping pong ball. The stream of air moves at high speed. As should be expected from Bernoulli's equation, this stream of air has a lower pressure than the stationary surrounding air.
- 145. • Activity! – Everyone can try with a bendy straw and ping pong ball. The stream of air moves at high speed. As should be expected from Bernoulli's equation, this stream of air has a lower pressure than the stationary surrounding air. If the ball starts to move to one side of the stream, the high- pressure of the stationary air pushes it back into the stream.
- 146. • Activity! (Optional) – Light candle directly behind box (non-flammable material) and try and blow out candle.
- 147. • Activity! (Optional) – Light candle directly behind tube / round container of about equal thickness (non- flammable material) and try and blow out candle.
- 148. • Activity! (Optional) – Light candle directly behind tube / round container of about equal thickness (non- flammable material) and try and blow out candle.
- 149. • Activity! (Optional) – Light candle directly behind tube / round container of about equal thickness (non- flammable material) and try and blow out candle.
- 150. • What happened? Why? – The air tended to stick to the curved surface of the bottle. This is called the Coanda effect.
- 151. • Quick Paper Airplane building contest! Copyright © 2010 Ryan P. Murphy
- 152. • Quick Paper Airplane building contest! – One piece 8 by 11, furthest flight wins, must be a plane with wings. Copyright © 2010 Ryan P. Murphy
- 153. • Quick Paper Airplane building contest! – One piece 8 by 11, furthest flight wins, must be a plane with wings. – Glider instructions on the next slide for those who need it. Copyright © 2010 Ryan P. Murphy
- 154. …
- 155. • Gas Laws and more available sheet.
- 156. • Gas Laws and more available sheet.
- 157. • Activity – Pressure and temperature. Copyright © 2010 Ryan P. Murphy
- 158. • Activity – Pressure and temperature. Copyright © 2010 Ryan P. Murphy
- 159. • Activity – Pressure and temperature. Copyright © 2010 Ryan P. Murphy
- 160. • Activity – Pressure and temperature. Copyright © 2010 Ryan P. Murphy
- 161. • Activity! Temp and Pressure.
- 162. • Activity! Temp and Pressure. – Record temperature inside bottle with cap off under normal atmospheric pressure.
- 163. • Activity! Temp and Pressure. – Record temperature inside bottle with cap off under normal atmospheric pressure. – Pump up bottle using “Fizz Keeper” as much as you can until it doesn’t create more pressure.
- 164. • Activity! Temp and Pressure. – Record temperature inside bottle with cap off under normal atmospheric pressure. – Pump up bottle using “Fizz Keeper” as much as you can until it doesn’t create more pressure. – Record temperature in bottle under pressure.
- 165. • Activity! Temp and Pressure. – Record temperature inside bottle with cap off under normal atmospheric pressure. – Pump up bottle using “Fizz Keeper” as much as you can until it doesn’t create more pressure. – Record temperature in bottle under pressure. – Observe the temperature as you unscrew the cap.
- 166. • Questions for the “Fizz Keeper Activity” – What was the temperature change? Copyright © 2010 Ryan P. Murphy
- 167. • Questions for the “Fizz Keeper Activity” – What was the temperature change? – How are pressure and temperature related? Copyright © 2010 Ryan P. Murphy
- 168. • Questions for the “Fizz Keeper Activity” – What was the temperature change? Copyright © 2010 Ryan P. Murphy
- 169. • Questions for the “Fizz Keeper Activity” – What was the temperature change? – The temperature increased a few degrees with increased pressure. Copyright © 2010 Ryan P. Murphy
- 170. • Questions for the “Fizz Keeper Activity” – How are pressure and temperature related? Copyright © 2010 Ryan P. Murphy
- 171. • Questions for the “Fizz Keeper Activity” – How are pressure and temperature related? – They are inversely proportional. When one goes up, the other goes down. Copyright © 2010 Ryan P. Murphy
- 172. Very Important! Record in Journal.
- 173. Copyright © 2010 Ryan P. Murphy
- 174. As pressure increases, temperature increases. Copyright © 2010 Ryan P. Murphy
- 175. As pressure increases, temperature increases. As pressure decreases, temperature decreases. Copyright © 2010 Ryan P. Murphy
- 176. • Pressure and temperature: Can you explain how this bird will continue to drink thinking about temperature and pressure? Copyright © 2010 Ryan P. Murphy
- 178. Answer: – Your body heat warms the fluid in the abdomen. Copyright © 2010 Ryan P. Murphy
- 179. Answer: – The heat increases the vapor pressure in the abdomen relative to the head (the reverse of what happens when you wet the head). Copyright © 2010 Ryan P. Murphy
- 180. Answer: – The fluid rises into the head in response to the pressure difference (moving from high pressure to low pressure). Copyright © 2010 Ryan P. Murphy
- 181. Answer: – The bird becomes top-heavy, and tips. Copyright © 2010 Ryan P. Murphy
- 182. Answer: – The bird becomes top-heavy, and tips. Copyright © 2010 Ryan P. Murphy
- 183. Temperature and Pressure Copyright © 2010 Ryan P. Murphy
- 184. Temperature and Pressure As temp rises, pressure rises Copyright © 2010 Ryan P. Murphy
- 185. Temperature and Pressure As temp rises, pressure rises As pressure rises, temp rises Copyright © 2010 Ryan P. Murphy
- 186. Temperature and Pressure As temp rises, pressure rises As pressure rises, temp rises Copyright © 2010 Ryan P. Murphy
- 187. Temperature and Pressure As temp rises, pressure rises As pressure rises, temp rises Copyright © 2010 Ryan P. Murphy
- 188. Temperature and Pressure As temp rises, pressure rises “Watch out” As pressure rises, temp rises Copyright © 2010 Ryan P. Murphy
- 189. Temperature and Pressure As temp rises, pressure rises “Watch out” As pressure rises, temp rises Copyright © 2010 Ryan P. Murphy
- 190. Temperature and Pressure As temp rises, pressure rises “Watch out” As pressure rises, temp rises “Watch out” Copyright © 2010 Ryan P. Murphy
- 191. +
- 202. +
- 203. +
- 205. • This photoshop job might look “Funny”.
- 206. • Caution! Graphic Images of burns / the dangers of pressure and temperature.
- 207. • The consequences of severe burns and explosions are not “funny”. Copyright © 2010 Ryan P. Murphy
- 211. The ideal gas law: PV = nRT (pressure times volume equals the number of molecules times the gas constant times temperature) Copyright © 2010 Ryan P. Murphy
- 212. The ideal gas law: PV = nRT (pressure times volume equals the number of molecules times the gas constant times temperature) Copyright © 2010 Ryan P. Murphy
- 213. The ideal gas law: PV = nRT (pressure times volume equals the number of molecules times the gas constant times temperature) Copyright © 2010 Ryan P. Murphy
- 214. The ideal gas law: PV = nRT (pressure times volume equals the number of molecules times the gas constant times temperature) Copyright © 2010 Ryan P. Murphy
- 215. The ideal gas law: PV = nRT (pressure times volume equals the number of molecules times the gas constant times temperature) Copyright © 2010 Ryan P. Murphy
- 216. The ideal gas law: PV = nRT (pressure times volume equals the number of molecules times the gas constant times temperature) Copyright © 2010 Ryan P. Murphy
- 217. The ideal gas law: PV = nRT (pressure times volume equals the number of molecules times the gas constant times temperature) Copyright © 2010 Ryan P. Murphy
- 218. P=Pressure V=Volume is equal to the.. n= Number of molecules R= Gas constant = 8.134 JK m T= Temperature Copyright © 2010 Ryan P. Murphy
- 219. P=Pressure V=Volume is equal to the.. n= Number of molecules R= Gas constant = 8.134 JK m T= Temperature Copyright © 2010 Ryan P. Murphy
- 220. P=Pressure V=Volume is equal to the.. n= Number of molecules R= Gas constant = 8.134 JK m T= Temperature Copyright © 2010 Ryan P. Murphy
- 221. P=Pressure V=Volume is equal to the.. n= Number of molecules R= Gas constant = 8.134 JK m T= Temperature Copyright © 2010 Ryan P. Murphy
- 222. P=Pressure V=Volume is equal to the.. n= Number of molecules R= Gas constant = 8.134 JK m T= Temperature Copyright © 2010 Ryan P. Murphy
- 223. P=Pressure V=Volume is equal to the.. n= Number of molecules R= Gas constant = 8.134 JK m T= Temperature Copyright © 2010 Ryan P. Murphy
- 224. P=Pressure V=Volume is equal to the.. n= Number of molecules R= Gas constant = 8.134 JK m T= Temperature Copyright © 2010 Ryan P. Murphy
- 225. • Video Link! (Optional) Khan Academy • Ideal Gas Law (Advanced) – http://www.khanacademy.org/video/ideal-gas- equation--pv-nrt?playlist=Chemistry
- 226. • Activity! Visiting Ideal Gas Law Simulator • http://www.7stones.com/Homepage/Publish er/Thermo1.html • How you can use this gas law to find…
- 227. • Activity! Visiting Ideal Gas Law Simulator • http://www.7stones.com/Homepage/Publish er/Thermo1.html • How you can use this gas law to find… – Calculating Volume of Ideal Gas: V = (nRT) ÷ P
- 228. • Activity! Visiting Ideal Gas Law Simulator • http://www.7stones.com/Homepage/Publish er/Thermo1.html • How you can use this gas law to find… – Calculating Volume of Ideal Gas: V = (nRT) ÷ P – Calculating Pressure of Ideal Gas: P = (nRT) ÷ V
- 229. • Activity! Visiting Ideal Gas Law Simulator • http://www.7stones.com/Homepage/Publish er/Thermo1.html • How you can use this gas law to find… – Calculating Volume of Ideal Gas: V = (nRT) ÷ P – Calculating Pressure of Ideal Gas: P = (nRT) ÷ V – Calculating moles of gas: n = (PV) ÷ (RT)
- 230. • Activity! Visiting Ideal Gas Law Simulator • http://www.7stones.com/Homepage/Publish er/Thermo1.html • How you can use this gas law to find… – Calculating Volume of Ideal Gas: V = (nRT) ÷ P – Calculating Pressure of Ideal Gas: P = (nRT) ÷ V – Calculating moles of gas: n = (PV) ÷ (RT) – Calculating gas temperature: T = (PV) ÷ (nR)
- 231. • Activity! Gas Law Simulator. • http://intro.chem.okstate.edu/1314F00/Lab oratory/GLP.htm • What happens to molecules when… – Temperature is increased. – Pressure is increased. – Volume is decreased. Copyright © 2010 Ryan P. Murphy
- 232. • Activity! Gas Law Simulator. • http://intro.chem.okstate.edu/1314F00/Lab oratory/GLP.htm • What happens to molecules when… – Temperature is increased. – Pressure is increased. – Volume is decreased. Copyright © 2010 Ryan P. Murphy
- 233. • Activity! Gas Law Simulator. • http://intro.chem.okstate.edu/1314F00/Lab oratory/GLP.htm • What happens to molecules when… – Temperature is increased. – Pressure is increased. – Volume is decreased. Copyright © 2010 Ryan P. Murphy
- 234. • Activity! Gas Law Simulator. • http://intro.chem.okstate.edu/1314F00/Lab oratory/GLP.htm • What happens to molecules when… – Temperature is increased. – Pressure is increased. – Volume is decreased. Copyright © 2010 Ryan P. Murphy
- 235. • Optional Class Quiz: The Quiz is difficult, but the correct answers are revealed which is the learning component. – Remember Kinetic Molecular Theory. – http://www.sciencegeek.net/Chemistry/taters/U nit5KMT.htm Copyright © 2010 Ryan P. Murphy
- 236. • Gas Laws and more available sheet.
- 237. • Activity / Happy Face Copyright © 2010 Ryan P. Murphy
- 238. • Activity / Demonstration! Copyright © 2010 Ryan P. Murphy
- 239. • Activity / Demonstration! – Blow up a balloon 1/8 of the way. Do not tie. the balloon! Copyright © 2010 Ryan P. Murphy
- 240. • Activity / Demonstration! – Blow up a balloon 1/8 of the way. Do not tie. the balloon! Copyright © 2010 Ryan P. Murphy
- 241. • Activity / Demonstration! – Blow up a balloon 1/8 of the way. Do not tie. the balloon! – Squeeze balloon in one hand and draw a small face on it with Sharpie marker (Works well if nose is the end of the balloon). Copyright © 2010 Ryan P. Murphy
- 242. • Activity / Demonstration! – Blow up a balloon 1/8 of the way. Do not tie. the balloon! – Squeeze balloon in one hand and draw a small face on it with Sharpie marker (Works well if nose is the end of the balloon). Copyright © 2010 Ryan P. Murphy
- 243. • Activity / Demonstration! – Blow up a balloon 1/8 of the way. Do not tie. the balloon! – Squeeze balloon in one hand and draw a small face on it with Sharpie marker (Works well if nose is the end of the balloon). Copyright © 2010 Ryan P. Murphy
- 244. • Activity / Demonstration! – Blow up a balloon 1/8 of the way. Do not tie. the balloon! – Squeeze balloon in one hand and draw a small face on it with Sharpie marker (Works well if nose is the end of the balloon). – Tie off balloon and face should shrink. Copyright © 2010 Ryan P. Murphy
- 245. • Activity / Demonstration! – Blow up a balloon 1/8 of the way. Do not tie. the balloon! – Squeeze balloon in one hand and draw a small face on it with Sharpie marker (Works well if nose is the end of the balloon). – Tie off balloon and face should shrink. – Release and then add pressure to one side of the balloon so that your face expands. Have fun for a bit! Copyright © 2010 Ryan P. Murphy
- 248. • Questions to the balloon poking. – How did the balloon and face change when you squished it? – How is pressure distributed when you squeeze the balloon? Copyright © 2010 Ryan P. Murphy
- 249. • Answer: By squeezing the balloon tightly, pressure is distributed equally in all directions. The face gets bigger evenly. Copyright © 2010 Ryan P. Murphy
- 250. • Video! The Blob. Trying to understand Pascal’s Law. – Can we create our own mini blob and send something flying with trash bags and textbooks. – http://www.youtube.com/watch?v=f2b8s4VxD60&feat ure=fvwrel Copyright © 2010 Ryan P. Murphy
- 251. • Video! The Blob. Trying to understand Pascal’s Law. – Can we create our own mini blob and send something flying with trash bags and textbooks. – http://www.youtube.com/watch?v=f2b8s4VxD60&feat ure=fvwrel Copyright © 2010 Ryan P. Murphy
- 252. Pascal's Law: If you apply pressure to fluids that are confined (or can’t flow anywhere), the fluids will then transmit (or send out) that same pressure in all directions at the same rate. Copyright © 2010 Ryan P. Murphy
- 253. Pascal's Law: If you apply pressure to fluids that are confined (or can’t flow anywhere), the fluids will then transmit (or send out) that same pressure in all directions at the same rate. Copyright © 2010 Ryan P. Murphy Cool Picture of a Gnome being squeezed and yelling something about Pascal in a different language.
- 254. Pascal's Law: If you apply pressure to fluids that are confined (or can’t flow anywhere), the fluids will then transmit (or send out) that same pressure in all directions at the same rate. Copyright © 2010 Ryan P. Murphy
- 257. • Hydraulics - The branch of applied science that deals with fluids in motion.
- 258. • Hydraulics - The branch of applied science that deals with fluids in motion.
- 261. • Don’t forget, air is also considered a fluid.
- 262. • Activity – Pascal’s Law and Hydraulics.
- 263. • Activity! Making a hydraulic syringe drive. Copyright © 2010 Ryan P. Murphy
- 264. • Activity! Making a hydraulic syringe drive. – Push syringe to bottom of tube on one side. Copyright © 2010 Ryan P. Murphy
- 265. • Activity! Making a hydraulic syringe drive. – Push syringe to bottom of tube on one side. – Dip end of syringe in water and pull to fill tube. Copyright © 2010 Ryan P. Murphy
- 266. • Activity! Making a hydraulic syringe drive. – Push syringe to bottom of tube on one side. – Dip end of syringe in water and pull to fill tube. – Attach hose to one side. Copyright © 2010 Ryan P. Murphy
- 267. • Activity! Making a hydraulic syringe drive. – Push syringe to bottom of tube on one side. – Dip end of syringe in water and pull to fill tube. – Attach hose to one side. – Depress syringe until water comes out of tube. Copyright © 2010 Ryan P. Murphy
- 268. • Activity! Making a hydraulic syringe drive. – Push syringe to bottom of tube on one side. – Dip end of syringe in water and pull to fill tube. – Attach hose to one side. – Depress syringe until water comes out of tube. – Attach other syringe that is depressed fully. Copyright © 2010 Ryan P. Murphy
- 269. • Activity! Making a hydraulic syringe drive. – Push syringe to bottom of tube on one side. – Dip end of syringe in water and pull to fill tube. – Attach hose to one side. – Depress syringe until water comes out of tube. – Attach other syringe that is depressed fully. – Push one side down at a time. Copyright © 2010 Ryan P. Murphy
- 270. • Questions to making a hydraulic syringe drive. – Draw / Sketch the hydraulic drive you created. – How is Pascal’s Law related to the hydraulic drive you just built? – Would it work better with oil, or with creamy peanut butter? Explain your answer using viscosity. Copyright © 2010 Ryan P. Murphy
- 271. • Questions to making a hydraulic syringe drive. – Draw / Sketch the hydraulic drive you created. – How is Pascal’s Law related to the hydraulic drive you just built? – Would it work better with oil, or with creamy peanut butter? Explain your answer using viscosity. Copyright © 2010 Ryan P. Murphy
- 272. • Questions to making a hydraulic syringe drive. – Draw / Sketch the hydraulic drive you created. – How is Pascal’s Law related to the hydraulic drive you just built? – Would it work better with oil, or with creamy peanut butter? Explain your answer using viscosity. Copyright © 2010 Ryan P. Murphy Viscosity: Resistance of liquid to flow.
- 273. • Questions to making a hydraulic syringe drive. – Draw / Sketch the hydraulic drive you created. – How is Pascal’s Law related to the hydraulic drive you just built? – Would it work better with oil, or with creamy peanut butter? Explain your answer using viscosity. Copyright © 2010 Ryan P. Murphy Viscosity: Resistance of liquid to flow. -High Viscosity = Difficult to flow.
- 274. • Questions to making a hydraulic syringe drive. – Draw / Sketch the hydraulic drive you created. – How is Pascal’s Law related to the hydraulic drive you just built? – Would it work better with oil, or with creamy peanut butter? Explain your answer using viscosity. Copyright © 2010 Ryan P. Murphy Viscosity: Resistance of liquid to flow. -High Viscosity = Difficult to flow. -Low Viscosity = Easy to flow.
- 275. • Questions to making a hydraulic syringe drive. – Draw / Sketch the hydraulic drive you created. – How is Pascal’s Law related to the hydraulic drive you just built? – Would it work better with oil, or with creamy peanut butter? Explain your answer using viscosity. Copyright © 2010 Ryan P. Murphy
- 276. • Questions to making a hydraulic syringe drive. – Draw / Sketch the hydraulic drive you created. Copyright © 2010 Ryan P. Murphy
- 277. • Questions to making a hydraulic syringe drive. – Draw / Sketch the hydraulic drive you created. Copyright © 2010 Ryan P. Murphy
- 278. • Questions to making a hydraulic syringe drive. – How is Pascal’s Law related to the hydraulic drive you just built? Copyright © 2010 Ryan P. Murphy
- 279. • Questions to making a hydraulic syringe drive. – How is Pascal’s Law related to the hydraulic drive you just built? – Answer: When the syringe is depressed, the fluid is sent out (transmitted) equally in all directions and flows through the tube to the syringe on the other side. Copyright © 2010 Ryan P. Murphy
- 280. • Questions to making a hydraulic syringe drive. – Would it work better with oil, or with creamy peanut butter? Explain your answer using viscosity. Copyright © 2010 Ryan P. Murphy
- 281. • Questions to making a hydraulic syringe drive. – Would it work better with oil, or with creamy peanut butter? Explain your answer using viscosity. – It would work better with oil because it has a lower viscosity (resistance to flow) Copyright © 2010 Ryan P. Murphy
- 282. • Questions to making a hydraulic syringe drive. – Would it work better with oil, or with creamy peanut butter? Explain your answer using viscosity. – It would work better with oil because it has a lower viscosity (resistance to flow) Copyright © 2010 Ryan P. Murphy Hydraulics, Learn more at… http://ffden- 2.phys.uaf.edu/212_spring2005.web.dir/annie_weber /page2.html
- 283. Viscosity: Resistance of liquid to flow. Copyright © 2010 Ryan P. Murphy
- 284. High Viscosity = Travels slow because of high resistance.
- 285. Low Viscosity = Travels fast because low resistance.
- 286. • Activity! What is more viscous? – Remember, Viscosity is resistance to flow. Copyright © 2010 Ryan P. Murphy
- 287. • Answer! The peanut butter doesn’t flow as much as the ketchup so it has more viscosity. Copyright © 2010 Ryan P. Murphy
- 288. • Viscosity Olympics Available Sheet
- 289. • Activity! The Condiment Olympics. – Official / ceremony / entrance of the condiments required. Volunteers needed to march each condiment into the classroom. – http://www.youtube.com/watch?v=EbHw8DBCXQ8 Copyright © 2010 Ryan P. Murphy
- 290. • Create the following spreadsheet in your journal. Condiment Finish Time Mustard Ketchup Jelly Maple Syrup (Fake) Chocolate Syrup Mystery Fluid Copyright © 2010 Ryan P. Murphy
- 291. • Create the following spreadsheet in your journal. Condiment Finish Time Mustard Ketchup Jelly Maple Syrup (Fake) Chocolate Syrup Mystery Fluid Copyright © 2010 Ryan P. Murphy
- 292. • Activity! Viscosity. – Lay tray on table.
- 293. • Activity! Viscosity. – Lay tray on table. – Place condiments at one side along a starting line.
- 294. • Activity! Viscosity. – Lay tray on table. – Place condiments at one side along a starting line. – Use textbooks or manually raise tray just off the vertical at start of race.
- 295. • Activity! Viscosity. – Lay tray on table. – Place condiments at one side along a starting line. – Use textbooks or manually raise tray just off the vertical at start of race. – Record the times each condiment takes to cross the finish line. (DNF = Did Not Finish) –I needed green text here to complete the Olympic colors.
- 296. • Activity! Viscosity. – Lay tray on table. – Place condiments at one side along a starting line. – Use textbooks or manually raise tray just off the vertical at start of race. – Record the times each condiment takes to cross the finish line. (DNF = Did Not Finish) –I needed green text here to complete the Olympic colors.
- 297. • Visual of Set-Up Top View Side View Start Finish
- 298. • Please graph your findings. You decide which graph will work the best. Pie Column Bar Line
- 299. • Please graph your findings. You decide which graph will work the best. Pie Column Bar Line
- 300. • Please graph your findings. You decide which graph will work the best. Pie Column Bar Line
- 301. • Please graph your findings. You decide which graph will work the best. Pie Column Bar Line
- 302. • Please graph your findings. You decide which graph will work the best. Pie Column Bar Line
- 303. • Please graph your findings. You decide which graph will work the best. Pie Column Bar LineA line graph could become confusing in this case
- 304. • Please graph your findings. You decide which graph will work the best. Pie Column Bar Line
- 305. • Please graph your findings. You decide which graph will work the best. Pie Column Bar Line
- 306. • Please graph your findings. You decide which graph will work the best. Pie Column Bar LineYou may begin creating your graph now.
- 307. • Viscosity Olympics Available Sheet
- 308. • Questions? – Which substance had the highest viscosity and why? – Which substance had the lowest viscosity and why? – Name five other things and describe their probable viscosity? Copyright © 2010 Ryan P. Murphy
- 309. • Graph of Possible Outcomes 0 0.5 1 1.5 2 2.5 3 3.5 Mustard Ketchup Jelly Maple Syrup Chocolate Syrup Mystery
- 310. • Viscosity Olympics Available Sheet
- 311. • Questions? – Which substance had the highest viscosity and why? Copyright © 2010 Ryan P. Murphy
- 312. • Questions? – Which substance had the highest viscosity and why? – Answer: Answers will vary based on the brand. Generally, the ketchup, mustard, and jelly was the slowest down the ramp and demonstrated most resistance to flow. Copyright © 2010 Ryan P. Murphy
- 313. • Questions? – Which substance had the lowest viscosity and why? Copyright © 2010 Ryan P. Murphy
- 314. • Questions? – Which substance had the lowest viscosity and why? – Answer: The real maple syrup had the lowest viscosity and traveled quickly down the ramp on to the floor almost immediately after putting it on the ramp. Copyright © 2010 Ryan P. Murphy
- 315. • Questions? – Name five other fluids and describe their probable viscosity? – Oil: Low viscosity – Peanut Butter: High Viscosity – Toothpaste: High Viscosity – Hair Gel: High Viscosity – Soda: Low Viscosity Copyright © 2010 Ryan P. Murphy
- 316. • Questions? – Name five other fluids and describe their probable viscosity? – Oil: Low viscosity Copyright © 2010 Ryan P. Murphy
- 317. • Questions? – Name five other fluids and describe their probable viscosity? – Oil: Low viscosity – Peanut Butter: High Viscosity – Toothpaste: High Viscosity – Hair Gel: High Viscosity – Soda: Low Viscosity Copyright © 2010 Ryan P. Murphy
- 318. • Questions? – Name five other fluids and describe their probable viscosity? – Oil: Low viscosity – Peanut Butter: High Viscosity – Toothpaste: High Viscosity – Hair Gel: High Viscosity – Soda: Low Viscosity Copyright © 2010 Ryan P. Murphy
- 319. • Questions? – Name five other fluids and describe their probable viscosity? – Oil: Low viscosity – Peanut Butter: High Viscosity – Toothpaste: High Viscosity – Hair Gel: High Viscosity – Soda: Low Viscosity Copyright © 2010 Ryan P. Murphy
- 320. • Questions? – Name five other fluids and describe their probable viscosity? – Oil: Low viscosity – Peanut Butter: High Viscosity – Toothpaste: High Viscosity – Hair Gel: High Viscosity – Soda: Low Viscosity Copyright © 2010 Ryan P. Murphy
- 321. • Questions? – Name five other fluids and describe their probable viscosity? – Oil: Low viscosity – Peanut Butter: High Viscosity – Toothpaste: High Viscosity – Hair Gel: High Viscosity – Soda: Low Viscosity Copyright © 2010 Ryan P. Murphy
- 322. • Questions? – Name five other fluids and describe their probable viscosity? – Oil: Low viscosity – Peanut Butter: High Viscosity – Toothpaste: High Viscosity – Hair Gel: High Viscosity – Soda: Low Viscosity Copyright © 2010 Ryan P. Murphy
- 323. • Questions? – Name five other fluids and describe their probable viscosity? – Oil: Low viscosity – Peanut Butter: High Viscosity – Toothpaste: High Viscosity – Hair Gel: High Viscosity – Soda: Low Viscosity Copyright © 2010 Ryan P. Murphy
- 324. • Questions? – Name five other fluids and describe their probable viscosity? – Oil: Low viscosity – Peanut Butter: High Viscosity – Toothpaste: High Viscosity – Hair Gel: High Viscosity – Soda: Low Viscosity Copyright © 2010 Ryan P. Murphy
- 325. • Questions? – Name five other fluids and describe their probable viscosity? – Oil: Low viscosity – Peanut Butter: High Viscosity – Toothpaste: High Viscosity – Hair Gel: High Viscosity – Soda: Low Viscosity Copyright © 2010 Ryan P. Murphy Viscosity: Learn more at… http://www.spacegrant.hawaii.edu/class_acts/ViscosityTe.h tml
- 326. • Video Link! Ants behaving like a viscous fluid. (Very optional but really neat) – https://www.youtube.com/watch?v=uZSqx0PJ 8XU&NR=1&feature=endscreen
- 327. • Activity! (Optional) Making Goop – Available Sheet
- 328. • Activity! (Optional) Making Goop • Directions in video and on next slide. • http://www.youtube.com/watch?v=48- DU0kQtPg
- 329. • Materials – Glue bottle (4oz) – 2 mixing bowls – Water – Mixing spoon – Measuring Cups – Borax – Measuring spoon – Sealable Bag
- 330. • Procedure: – 1.) Squeeze glue into bowl. – 2.) Fill glue bottle with water, cap, mix, and pour into the glue in bowl. – 3.) Stir and add desired food coloring. – 4.) Set that bowl aside. – 5.) In new bowl mix 1 cup of water with 1 tablespoon of borax and stir. – 6.) Add 1/3 a cup of borax and water mixture into a bowl and stir. – 7.) Slowly add the contents from the glue bowl into the borax bowl while you stir. – 8.) Pick up goop and work it with your hands. Put in plastic bag and clean up area. – 9.)Once area is clean you can play with goop.
- 331. • Goop is a polymer you can make from white glue and borax. – Borax is a cleaning agent and natural mineral composed of sodium, boron, oxygen and water. – The Elmer’s glue is a long-chained polymer (Poly Vinyl Acetate), meaning it is a set of molecules that are linked together in a long chain. – When added together, the borate ions bond with water molecules. These long polymers link together to form a matrix that is not very strong. – This why goop is stretchable and considered a Non-Newtonian Fluid. High Viscosity.
- 332. Archimedes Principle – Any Guesses? Copyright © 2010 Ryan P. Murphy
- 333. Archimedes Principle: A body that is submerged in a fluid is buoyed up by a force equal in magnitude to the weight of the fluid that is displaced. Copyright © 2010 Ryan P. Murphy
- 334. Archimedes Principle: A body that is submerged in a fluid is buoyed up by a force equal in magnitude to the weight of the fluid that is displaced. Copyright © 2010 Ryan P. Murphy
- 335. Archimedes Principle: A body that is submerged in a fluid is buoyed up by a force equal in magnitude to the weight of the fluid that is displaced. Copyright © 2010 Ryan P. Murphy
- 336. Archimedes Principle: A body that is submerged in a fluid is buoyed up by a force equal in magnitude to the weight of the fluid that is displaced. Copyright © 2010 Ryan P. Murphy
- 337. Archimedes Principle: A body that is submerged in a fluid is buoyed up by a force equal in magnitude to the weight of the fluid that is displaced. Copyright © 2010 Ryan P. Murphy Boat must weigh less than this much water to float.
- 338. Copyright © 2010 Ryan P. Murphy
- 339. Copyright © 2010 Ryan P. Murphy
- 340. Copyright © 2010 Ryan P. Murphy
- 341. Buoyancy: Buoyancy force is equal to the weight of fluid displaced by the body. Copyright © 2010 Ryan P. Murphy
- 342. • If your boat doesn’t displace more water than it weighs, your boat will sink. Copyright © 2010 Ryan P. Murphy
- 349. • Density: How much mass is contained in a given volume. We use grams/cm3 – (grams per cubic centimeter) Copyright © 2010 Ryan P. Murphy
- 350. • Density: How much mass is contained in a given volume. We use grams/cm3 – (grams per cubic centimeter) – Density = Mass divided by volume Copyright © 2010 Ryan P. Murphy
- 351. • Density: How much mass is contained in a given volume. We use grams/cm3 – (grams per cubic centimeter) – Density = Mass divided by volume Copyright © 2010 Ryan P. Murphy Mass D = ------------- = grams/cm3 Volume
- 352. • Please determine the densities of the following characters. Who is most dense? Donkey Kong M = 15 g V = 30 cm3 Yoshi M = 6g V = 8 cm3 Mario M = 8g V = 10cm3 Goomba M = 8g V = 6 cm3
- 353. • Please determine the densities of the following characters. Who is most dense? Donkey Kong. 5 g/cm3 Yoshi .75 g/cm3 Mario .8 g/cm3 Goomba 1.3 g/cm3
- 354. • Please determine the densities of the following characters. Who is most dense? Donkey Kong. .5 g/cm3 Yoshi .75 g/cm3 Mario .8 g/cm3 Goomba 1.3 g/cm3
- 355. • Please determine the densities of the following characters. Who is most dense? Donkey Kong. .5 g/cm3 Yoshi .75 g/cm3 Mario .8 g/cm3 Goomba 1.3 g/cm3
- 356. • Please determine the densities of the following characters. Who is most dense? Donkey Kong. .5 g/cm3 Yoshi .75 g/cm3 Mario .8 g/cm3 Goomba 1.3 g/cm3
- 357. • Please determine the densities of the following characters. Who is most dense? Donkey Kong. .5 g/cm3 Yoshi .75 g/cm3 Mario .8 g/cm3 Goomba 1.3 g/cm3
- 358. • Please determine the densities of the following characters. Who is most dense? Donkey Kong. .5 g/cm3 Yoshi .75 g/cm3 Mario .8 g/cm3 Goomba 1.3 g/cm3
- 359. • Please determine the densities of the following characters. Who is most dense? Donkey Kong. .5 g/cm3 Yoshi .75 g/cm3 Mario .8 g/cm3 Goomba 1.3 g/cm3
- 360. • Please determine the densities of the following characters. Who is most dense? Donkey Kong. .5 g/cm3 Yoshi .75 g/cm3 Mario .8 g/cm3 Goomba 1.3 g/cm3
- 361. • Which one will sink in water? Donkey Kong. .5 g/cm3 Yoshi .75 g/cm3 Mario .8 g/cm3 Goomba 1.3 g/cm3
- 371. • Volume and Density Available Sheet. – Additional classwork / homework
- 372. • Buoyancy Simulator: http://phet.colorado.edu/en/simulation/buo yancy • Density Simulator • http://phet.colorado.edu/en/simulation/den sity
- 373. • Activity! Creating a boat using Archimedes Principle. Copyright © 2010 Ryan P. Murphy
- 380. • Activity – Creating a boat with a chunk of clay. Copyright © 2010 Ryan P. Murphy
- 381. • Activity – Creating a boat with a chunk of clay. – Everyone will get the same amount of clay in grams (200 grams of clay). Copyright © 2010 Ryan P. Murphy
- 382. • Activity – Creating a boat with a chunk of clay. – Everyone will get the same amount of clay in grams (200 grams of clay). – Weights will be placed on the boat. Copyright © 2010 Ryan P. Murphy
- 383. • Activity – Creating a boat with a chunk of clay. – Everyone will get the same amount of clay in grams (200 grams of clay). – Weights will be placed on the boat. – Your grade depends on the buoyancy of your boat. Copyright © 2010 Ryan P. Murphy
- 384. • Activity – Creating a boat with a chunk of clay. – Everyone will get the same amount of clay in grams (200 grams of clay). – Weights will be placed on the boat. – Your grade depends on the buoyancy of your boat. – Must be stable enough to support weight. Copyright © 2010 Ryan P. Murphy
- 385. • Possible hull designs.
- 386. • Possible hull designs.
- 387. • Possible hull designs.
- 388. • Possible hull designs.
- 389. • Possible hull designs.
- 390. • How does a submarine dive and then rise?
- 391. • How does a submarine dive and then rise?
- 392. • How does a submarine dive and then rise? • Answer: The buoyancy of a submarine can be changed by pumping water into the main ballast tanks and removing air (sinks) or pumping air into the tanks and releasing the water (floats).
- 393. • Activity - Making a Cartesian Diver – – Provided hand out with directions and questions to be answered in journal. Copyright © 2010 Ryan P. Murphy
- 394. • Activity - Making a Cartesian Diver – – Provided hand out with directions and questions to be answered in journal. Copyright © 2010 Ryan P. Murphy
- 395. • Optional: Make a Squidy – Watch video and create in real time / pausing. – http://www.youtube.com/watch?v=5LLwTIkRZAU
- 396. • Cartesian Diver Available Sheet Learn more at… http://courses.education.illinois.edu/ci2 41-science- sp95/resources/philotoy/philotoy.html
- 402. • Please write a short essay in your journal – Explain the Cartesian Diver using Archimedes Principle, Pascal’s Law, and Boyle’s Law within your response. Copyright © 2010 Ryan P. Murphy
- 403. • Please write a short essay in your journal – Explain the Cartesian Diver using Archimedes Principle, Pascal’s Law, and Boyle’s Law within your response. • 1st paragraph Introduce Cartesian diver and three laws. Copyright © 2010 Ryan P. Murphy
- 404. • Please write a short essay in your journal – Explain the Cartesian Diver using Archimedes Principle, Pascal’s Law, and Boyle’s Law within your response. • 1st paragraph Introduce Cartesian diver and three laws. • 2nd paragraph (Pascal’s Law) Copyright © 2010 Ryan P. Murphy
- 405. • Please write a short essay in your journal – Explain the Cartesian Diver using Archimedes Principle, Pascal’s Law, and Boyle’s Law within your response. • 1st paragraph Introduce Cartesian diver and three laws. • 2nd paragraph (Pascal’s Law) • 3rd paragraph (Boyles Law) Copyright © 2010 Ryan P. Murphy
- 406. • Please write a short essay in your journal – Explain the Cartesian Diver using Archimedes Principle, Pascal’s Law, and Boyle’s Law within your response. • 1st paragraph Introduce Cartesian diver and three laws. • 2nd paragraph (Pascal’s Law) • 3rd paragraph (Boyles Law) • 4th paragraph (Archimedes Principle) Copyright © 2010 Ryan P. Murphy
- 407. • Please write a short essay in your journal – Explain the Cartesian Diver using Archimedes Principle, Pascal’s Law, and Boyle’s Law within your response. • 1st paragraph Introduce Cartesian diver and three laws. • 2nd paragraph (Pascal’s Law) • 3rd paragraph (Boyles Law) • 4th paragraph (Archimedes Principle) • 5th paragraph (Conclusion) Copyright © 2010 Ryan P. Murphy
- 408. • Answer – It’s all about the gas inside the eye dropper. – What happens to it when the bottle is squeezed? Copyright © 2010 Ryan P. Murphy
- 409. • Your response should include… Copyright © 2010 Ryan P. Murphy
- 410. • Your response should include… – When you squeeze the bottle, (increase pressure). Copyright © 2010 Ryan P. Murphy
- 411. • Your response should include… – When you squeeze the bottle, (increase pressure). – The pressure is distributed equally in all directions (Pascal’s Law). Copyright © 2010 Ryan P. Murphy
- 412. • Your response should include… – When you squeeze the bottle, (increase pressure). – The pressure is distributed equally in all directions (Pascal’s Law). – The increase in pressure decreases the volume of the gas inside the eye dropper (Boyles Law). Copyright © 2010 Ryan P. Murphy
- 413. • Your response should include… – When you squeeze the bottle, (increase pressure). – The pressure is distributed equally in all directions (Pascal’s Law). – The increase in pressure decreases the volume of the gas inside the eye dropper (Boyles Law). – The decreased volume displaces less water so the diver is less buoyant and sinks (Archimedes Principle). Copyright © 2010 Ryan P. Murphy
- 415. The Cartesian Diver is an experiment that demonstrates three important science concepts. Pascal’s Law, Boyles Law, and Archimedes Principle all help to explain how a Cartesian Diver works. When the bottle is squeezed, the fluid transmits a pressure equally in all directions. This is Pascal’s Law. The pressure worked on the eye dropper as well as the plastic bottle. When the bottle was squeezed, the air bubble inside the eye dropper got smaller. This was an example of Boyles Law, that when pressure is exerted on a gas, its volume will decrease. The decrease in volume of the gas caused the diver to displace less water than before. Under Archimedes Principle, the diver should sink which it did. When pressure was released, the volume of the gas increased, more water was displaced and the diver rose to the surface. All three of these important concepts working together are represented in a Cartesian Diver.
- 416. The Cartesian Diver is an experiment that demonstrates three important science concepts. Pascal’s Law, Boyles Law, and Archimedes Principle all help to explain how a Cartesian Diver works. When the bottle is squeezed, the fluid transmits a pressure equally in all directions. This is Pascal’s Law. The pressure worked on the eye dropper as well as the plastic bottle. When the bottle was squeezed, the air bubble inside the eye dropper got smaller. This was an example of Boyles Law, that when pressure is exerted on a gas, its volume will decrease. The decrease in volume of the gas caused the diver to displace less water than before. Under Archimedes Principle, the diver should sink which it did. When pressure was released, the volume of the gas increased, more water was displaced and the diver rose to the surface. All three of these important concepts working together are represented in a Cartesian Diver.
- 417. The Cartesian Diver is an experiment that demonstrates three important science concepts. Pascal’s Law, Boyles Law, and Archimedes Principle all help to explain how a Cartesian Diver works. When the bottle is squeezed, the fluid transmits a pressure equally in all directions. This is Pascal’s Law. The pressure worked on the eye dropper as well as the plastic bottle. When the bottle was squeezed, the air bubble inside the eye dropper got smaller. This was an example of Boyles Law, that when pressure is exerted on a gas, its volume will decrease. The decrease in volume of the gas caused the diver to displace less water than before. Under Archimedes Principle, the diver should sink which it did. When pressure was released, the volume of the gas increased, more water was displaced and the diver rose to the surface. All three of these important concepts working together are represented in a Cartesian Diver.
- 418. The Cartesian Diver is an experiment that demonstrates three important science concepts. Pascal’s Law, Boyles Law, and Archimedes Principle all help to explain how a Cartesian Diver works. When the bottle is squeezed, the fluid transmits a pressure equally in all directions. This is Pascal’s Law. The pressure worked on the eye dropper as well as the plastic bottle. When the bottle was squeezed, the air bubble inside the eye dropper got smaller. This was an example of Boyles Law, that when pressure is exerted on a gas, its volume will decrease. The decrease in volume of the gas caused the diver to displace less water than before. Under Archimedes Principle, the diver should sink which it did. When pressure was released, the volume of the gas increased, more water was displaced and the diver rose to the surface. All three of these important concepts working together are represented in a Cartesian Diver.
- 419. The Cartesian Diver is an experiment that demonstrates three important science concepts. Pascal’s Law, Boyles Law, and Archimedes Principle all help to explain how a Cartesian Diver works. When the bottle is squeezed, the fluid transmits a pressure equally in all directions. This is Pascal’s Law. The pressure worked on the eye dropper as well as the plastic bottle. When the bottle was squeezed, the air bubble inside the eye dropper got smaller. This was an example of Boyles Law, that when pressure is exerted on a gas, its volume will decrease. The decrease in volume of the gas caused the diver to displace less water than before. Under Archimedes Principle, the diver should sink which it did. When pressure was released, the volume of the gas increased, more water was displaced and the diver rose to the surface. All three of these important concepts working together are represented in a Cartesian Diver.
- 420. The Cartesian Diver is an experiment that demonstrates three important science concepts. Pascal’s Law, Boyles Law, and Archimedes Principle all help to explain how a Cartesian Diver works. When the bottle is squeezed, the fluid transmits a pressure equally in all directions. This is Pascal’s Law. The pressure worked on the eye dropper as well as the plastic bottle. When the bottle was squeezed, the air bubble inside the eye dropper got smaller. This was an example of Boyles Law, that when pressure is exerted on a gas, its volume will decrease. The decrease in volume of the gas caused the diver to displace less water than before. Under Archimedes Principle, the diver should sink which it did. When pressure was released, the volume of the gas increased, more water was displaced and the diver rose to the surface. All three of these important concepts working together are represented in a Cartesian Diver.
- 421. The Cartesian Diver is an experiment that demonstrates three important science concepts. Pascal’s Law, Boyles Law, and Archimedes Principle all help to explain how a Cartesian Diver works. When the bottle is squeezed, the fluid transmits a pressure equally in all directions. This is Pascal’s Law. The pressure worked on the eye dropper as well as the plastic bottle. When the bottle was squeezed, the air bubble inside the eye dropper got smaller. This was an example of Boyles Law, that when pressure is exerted on a gas, its volume will decrease. The decrease in volume of the gas caused the diver to displace less water than before. Under Archimedes Principle, the diver should sink which it did. When pressure was released, the volume of the gas increased, more water was displaced and the diver rose to the surface. All three of these important concepts working together are represented in a Cartesian Diver.
- 422. • Try and be the first to figure out the hidden picture beneath the boxes. – Raise your hand when you think you know, you only get one guess. Copyright © 2010 Ryan P. Murphy
- 433. • Try and be the first to figure out the hidden picture beneath the boxes. – Raise your hand when you think you know, you only get one guess. Copyright © 2010 Ryan P. Murphy
- 446. • Try and be the first to figure out the hidden picture beneath the boxes. – Raise your hand when you think you know, you only get one guess. Copyright © 2010 Ryan P. Murphy
- 447. Copyright © 2010 Ryan P. Murphy
- 448. Copyright © 2010 Ryan P. Murphy
- 449. Copyright © 2010 Ryan P. Murphy
- 450. Copyright © 2010 Ryan P. Murphy
- 451. Copyright © 2010 Ryan P. Murphy
- 452. Copyright © 2010 Ryan P. Murphy
- 453. Copyright © 2010 Ryan P. Murphy
- 454. Copyright © 2010 Ryan P. Murphy
- 455. Copyright © 2010 Ryan P. Murphy
- 456. Copyright © 2010 Ryan P. Murphy “Would you like me to show you some gas laws?”
- 457. • Try and be the first to figure out the hidden picture beneath the boxes. – Raise your hand when you think you know, you only get one guess. Copyright © 2010 Ryan P. Murphy
- 471. • You should be close to page 5 and can now complete the Cartesian Diver question on page 6.
- 472. • You can now provide text in the white space and then neatly color the following.
- 490. • “AYE” Advance Your Exploration ELA and Literacy Opportunity Worksheet – Visit some of the many provided links or.. – Articles can be found at (w/ membership to NABT and NSTA) • http://www.nabt.org/websites/institution/index.php?p= 1 • http://learningcenter.nsta.org/browse_journals.aspx?j ournal=tst Please visit at least one of the “learn more” educational links provided in this unit and complete this worksheet
- 491. • “AYE” Advance Your Exploration ELA and Literacy Opportunity Worksheet – Visit some of the many provided links or.. – Articles can be found at (w/ membership to and NSTA) • http://www.sciencedaily.com/ • http://www.sciencemag.org/ • http://learningcenter.nsta.org/browse_journals.aspx?jo urnal=tst
- 494. http://sciencepowerpoint.com/Energy_Topics_Unit.html Areas of Focus within The Matter, Energy, and the Environment Unit. There is no such thing as a free lunch, Matter, Dark Matter, Elements and Compounds, States of Matter, Solids, Liquids, Gases, Plasma, Law Conservation of Matter, Physical Change, Chemical Change, Gas Laws, Charles Law, Avogadro’s Law, Ideal Gas Law, Pascal’s Law, Viscosity, Archimedes Principle, Buoyancy, Seven Forms of Energy, Nuclear Energy, Electromagnet Spectrum, Waves / Wavelengths, Light (Visible Light), Refraction, Diffraction, Lens, Convex / Concave, Radiation, Electricity, Lightning, Static Electricity, Magnetism, Coulomb’s Law, Conductors, Insulators, Semi-conductors, AC and DC current, Amps, Watts, Resistance, Magnetism, Faraday’s Law, Compass, Relativity, Einstein, and E=MC2, Energy, First Law of Thermodynamics, Second Law of Thermodynamics, Third Law of Thermodynamics, Industrial Processes, Environmental Studies, The 4 R’s, Sustainability, Human Population Growth, Carrying Capacity, Green Design, Renewable Forms of Energy.
- 504. • Please visit the links below to learn more about each of the units in this curriculum – These units take me about four years to complete with my students in grades 5-10. Earth Science Units Extended Tour Link and Curriculum Guide Geology Topics Unit http://sciencepowerpoint.com/Geology_Unit.html Astronomy Topics Unit http://sciencepowerpoint.com/Astronomy_Unit.html Weather and Climate Unit http://sciencepowerpoint.com/Weather_Climate_Unit.html Soil Science, Weathering, More http://sciencepowerpoint.com/Soil_and_Glaciers_Unit.html Water Unit http://sciencepowerpoint.com/Water_Molecule_Unit.html Rivers Unit http://sciencepowerpoint.com/River_and_Water_Quality_Unit.html = Easier = More Difficult = Most Difficult 5th – 7th grade 6th – 8th grade 8th – 10th grade
- 505. Physical Science Units Extended Tour Link and Curriculum Guide Science Skills Unit http://sciencepowerpoint.com/Science_Introduction_Lab_Safety_Metric_Methods. html Motion and Machines Unit http://sciencepowerpoint.com/Newtons_Laws_Motion_Machines_Unit.html Matter, Energy, Envs. Unit http://sciencepowerpoint.com/Energy_Topics_Unit.html Atoms and Periodic Table Unit http://sciencepowerpoint.com/Atoms_Periodic_Table_of_Elements_Unit.html Life Science Units Extended Tour Link and Curriculum Guide Human Body / Health Topics http://sciencepowerpoint.com/Human_Body_Systems_and_Health_Topics_Unit.html DNA and Genetics Unit http://sciencepowerpoint.com/DNA_Genetics_Unit.html Cell Biology Unit http://sciencepowerpoint.com/Cellular_Biology_Unit.html Infectious Diseases Unit http://sciencepowerpoint.com/Infectious_Diseases_Unit.html Taxonomy and Classification Unit http://sciencepowerpoint.com/Taxonomy_Classification_Unit.html Evolution / Natural Selection Unit http://sciencepowerpoint.com/Evolution_Natural_Selection_Unit.html Botany Topics Unit http://sciencepowerpoint.com/Plant_Botany_Unit.html Ecology Feeding Levels Unit http://sciencepowerpoint.com/Ecology_Feeding_Levels_Unit.htm Ecology Interactions Unit http://sciencepowerpoint.com/Ecology_Interactions_Unit.html Ecology Abiotic Factors Unit http://sciencepowerpoint.com/Ecology_Abiotic_Factors_Unit.html
- 506. • The entire four year curriculum can be found at... http://sciencepowerpoint.com/ Please feel free to contact me with any questions you may have. Thank you for your interest in this curriculum. Sincerely, Ryan Murphy M.Ed www.sciencepowerpoint@gmail.com
