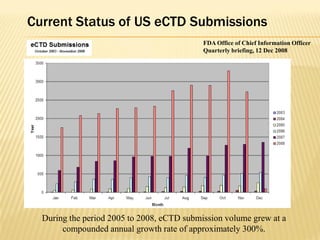
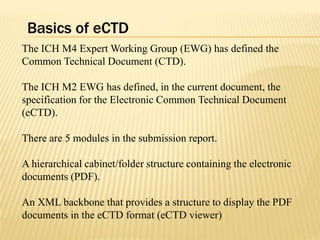
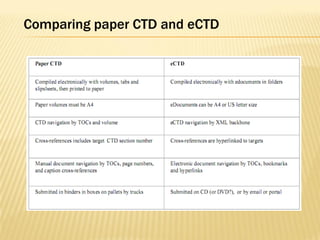
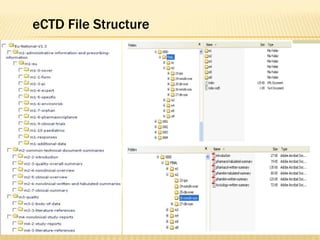
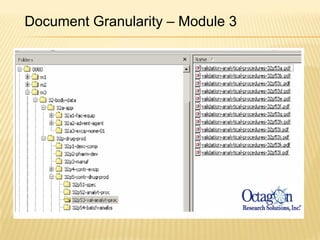
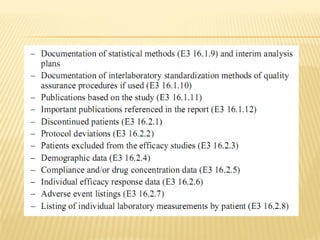
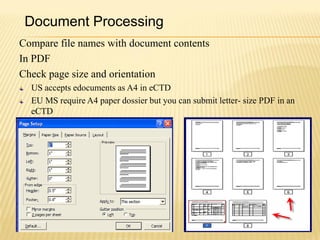
The document outlines the Common Technical Document (CTD) and its electronic variant (eCTD), adopted by the FDA and other global regulatory agencies for drug submissions. It highlights the eCTD's growing adoption in the U.S. and Europe, detailing the structure, submission modules, and challenges faced in transitioning from paper to electronic formats. Key points include the significant increase in eCTD submissions from 2005 to 2008, the technological and procedural requirements for eCTD compliance, and the anticipated shift towards a paperless submission landscape.