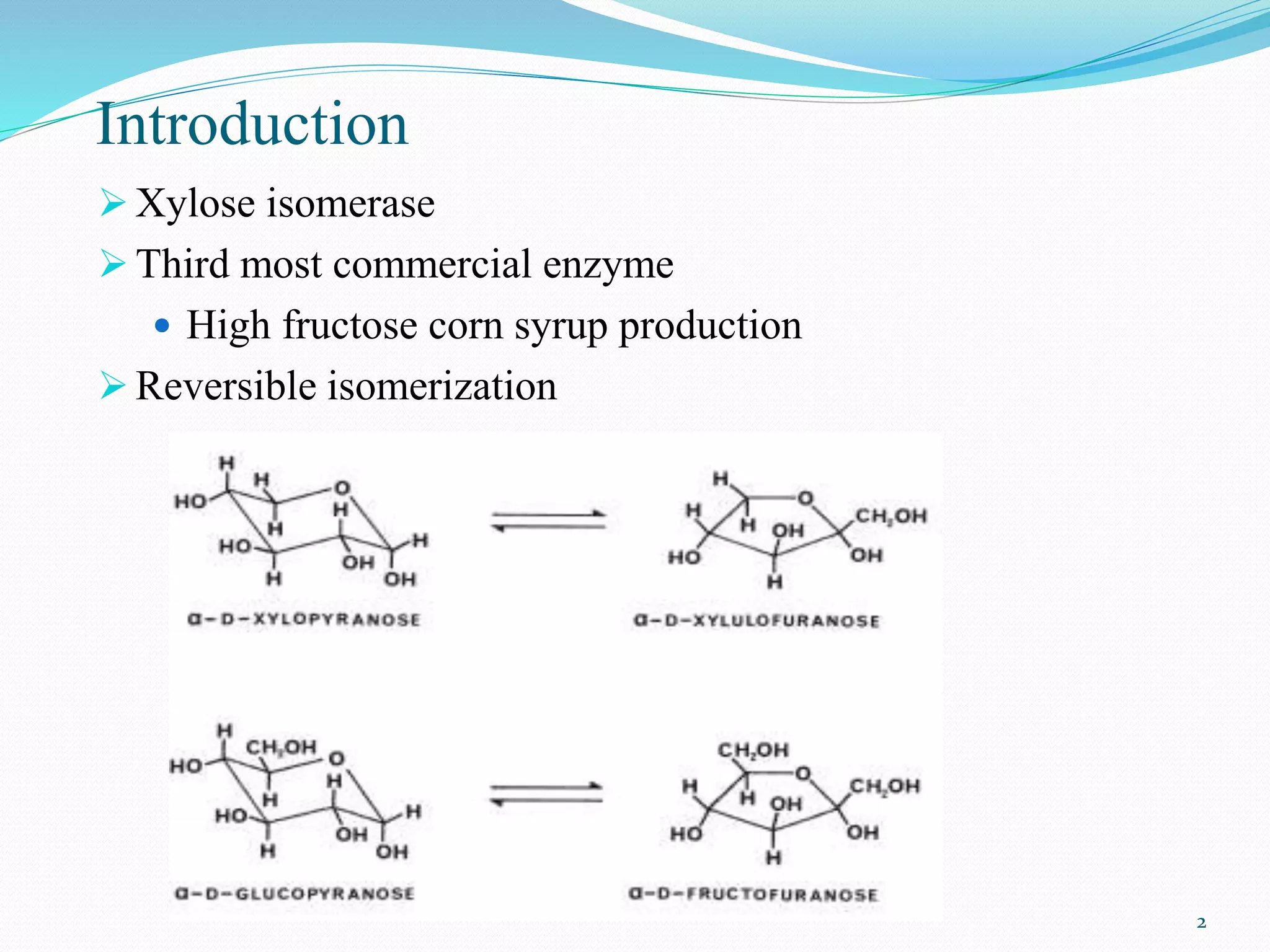
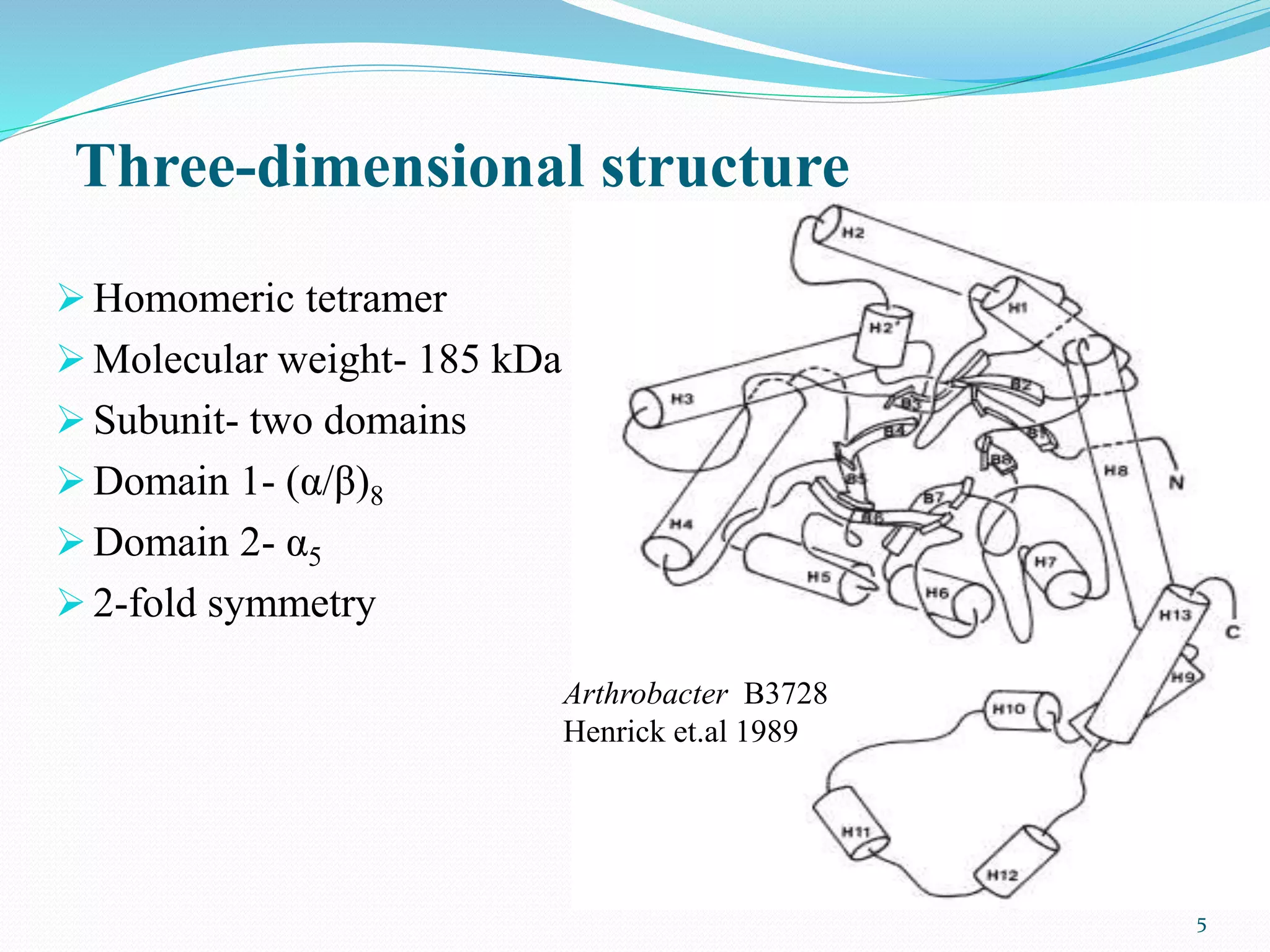
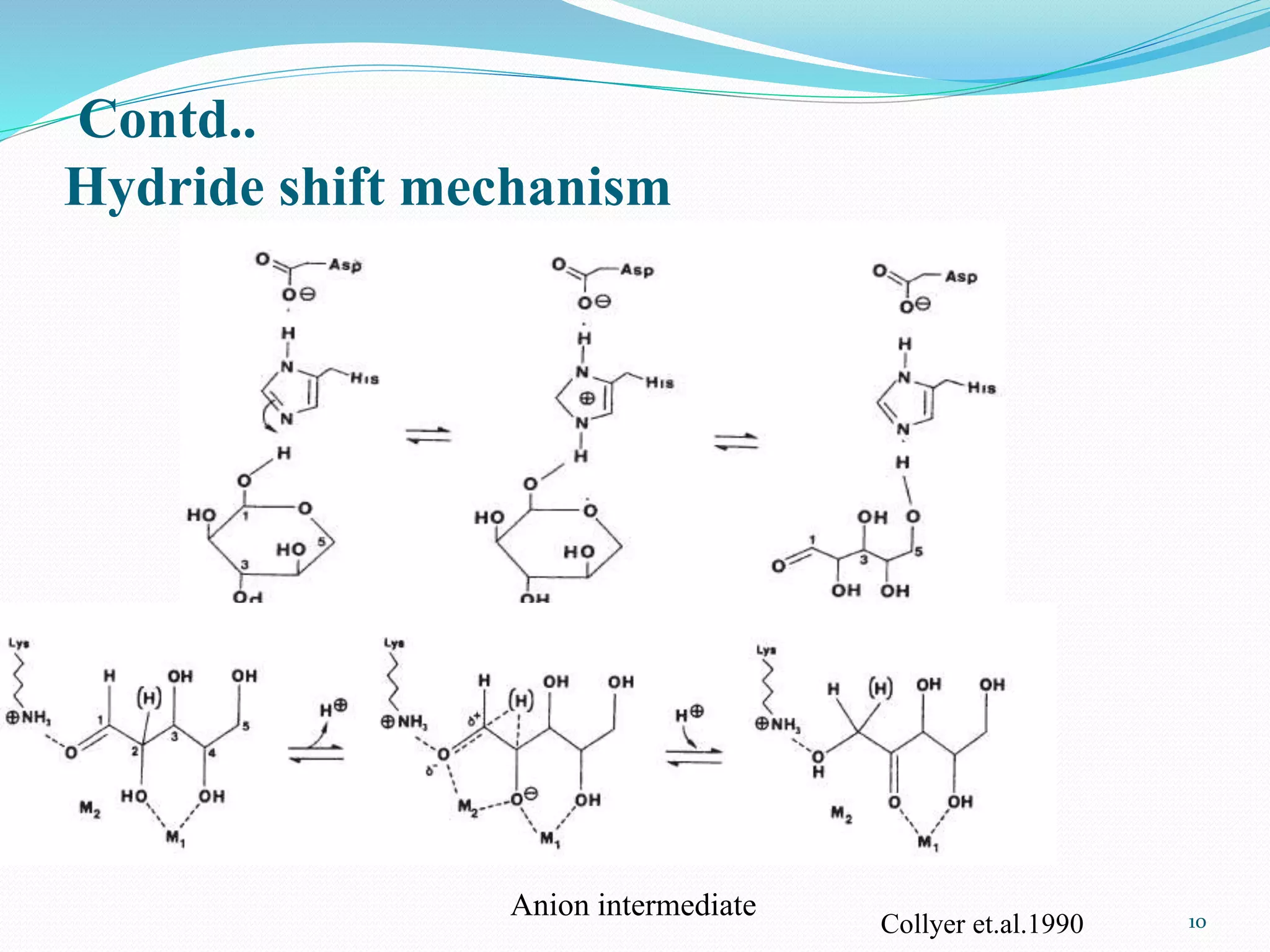
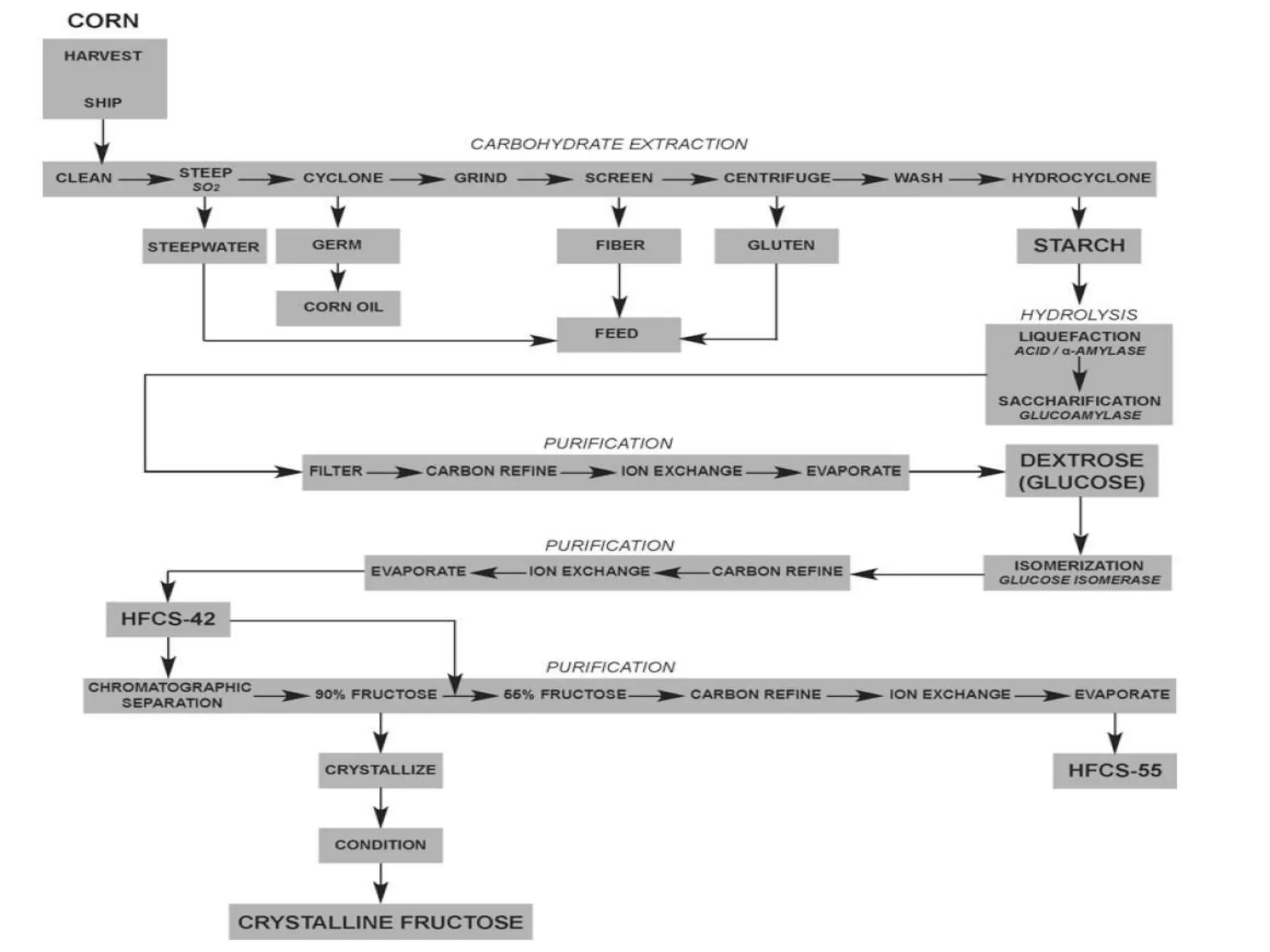
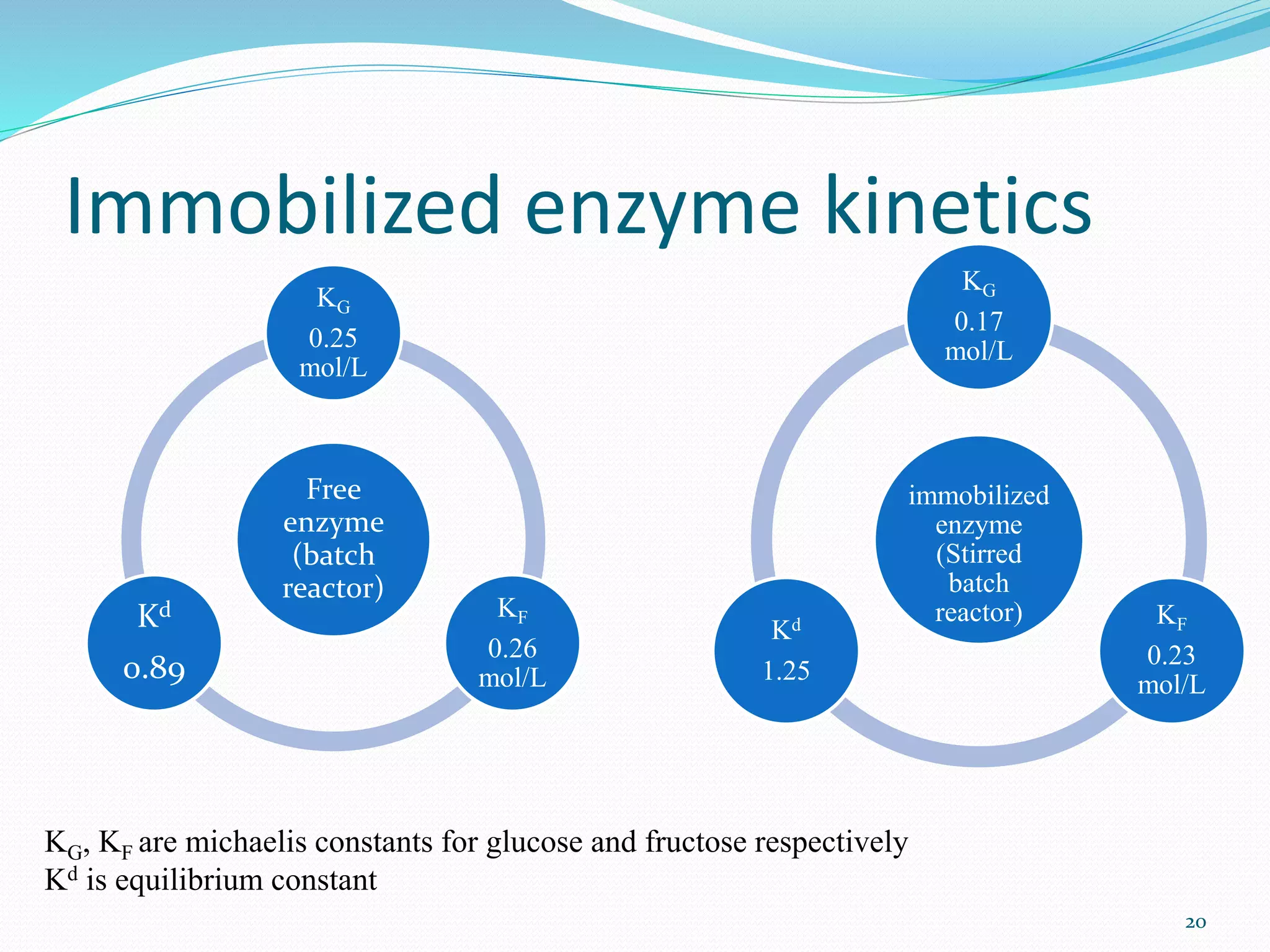
Xylose isomerase is the third most commercially important enzyme used in the production of high fructose corn syrup. It catalyzes the reversible isomerization of D-xylose and D-glucose. Glucose isomerase is a tetrameric protein containing two metal binding sites and uses a hydride shift mechanism to convert glucose to fructose. It is produced commercially using various microorganisms like Bacillus, Streptomyces, and Candida species. Glucose isomerase is immobilized and used for continuous production of high fructose corn syrup from glucose through isomerization in industrial processes.