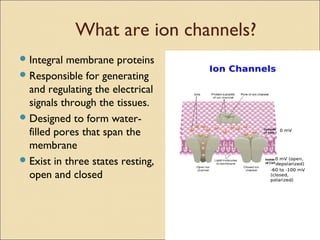
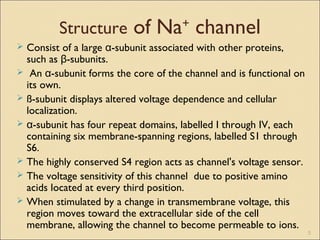
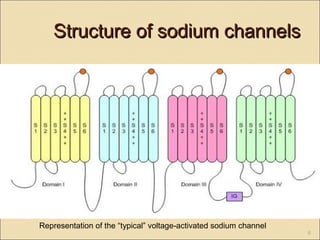
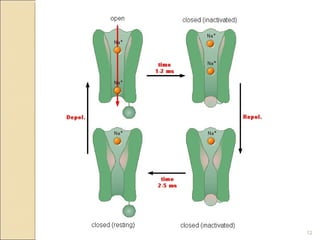
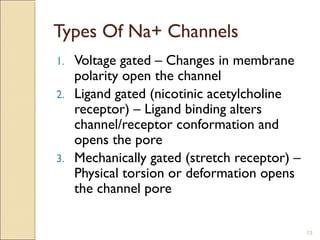
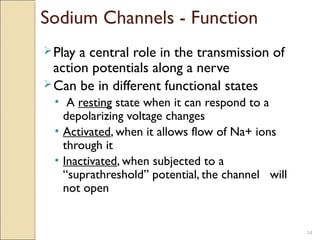
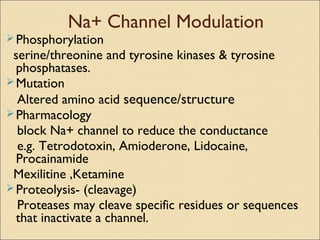
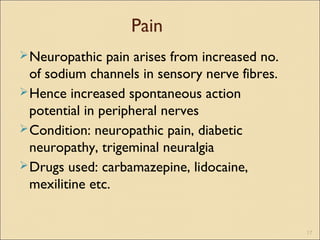
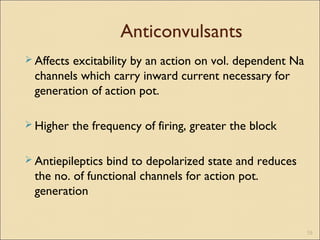
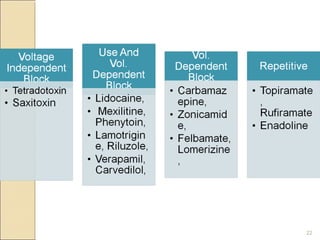
The document provides an overview of sodium channels, detailing their molecular structure, gating mechanisms, various types, and their functions in generating electrical signals in tissues. It discusses the modulation of sodium channels through phosphorylation and pharmacological agents, highlighting their therapeutic applications in conditions such as epilepsy, neuropathic pain, and local anesthesia. The document also covers the effects of sodium channel blockers on excitability and their role in heart rhythm management.