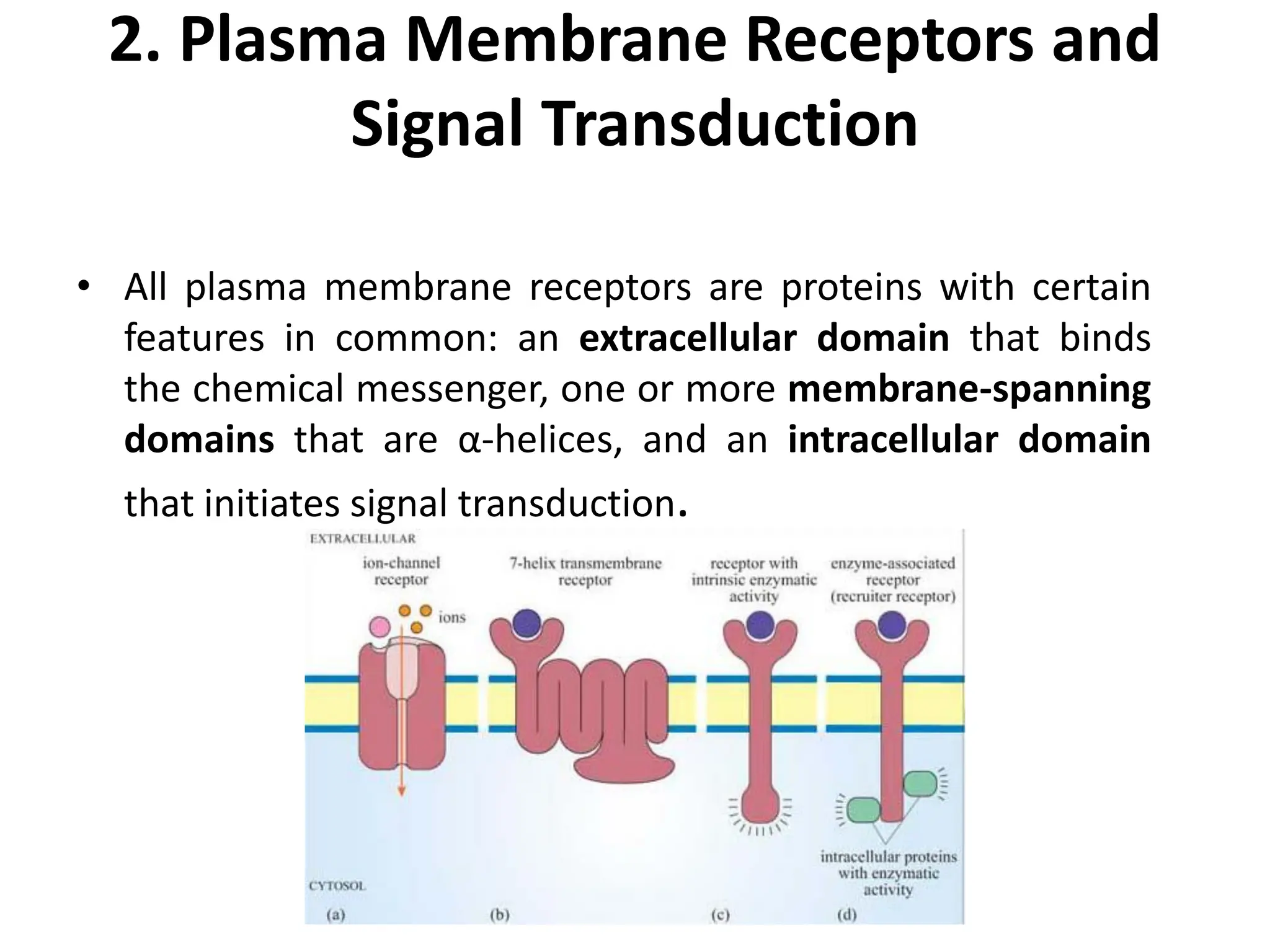
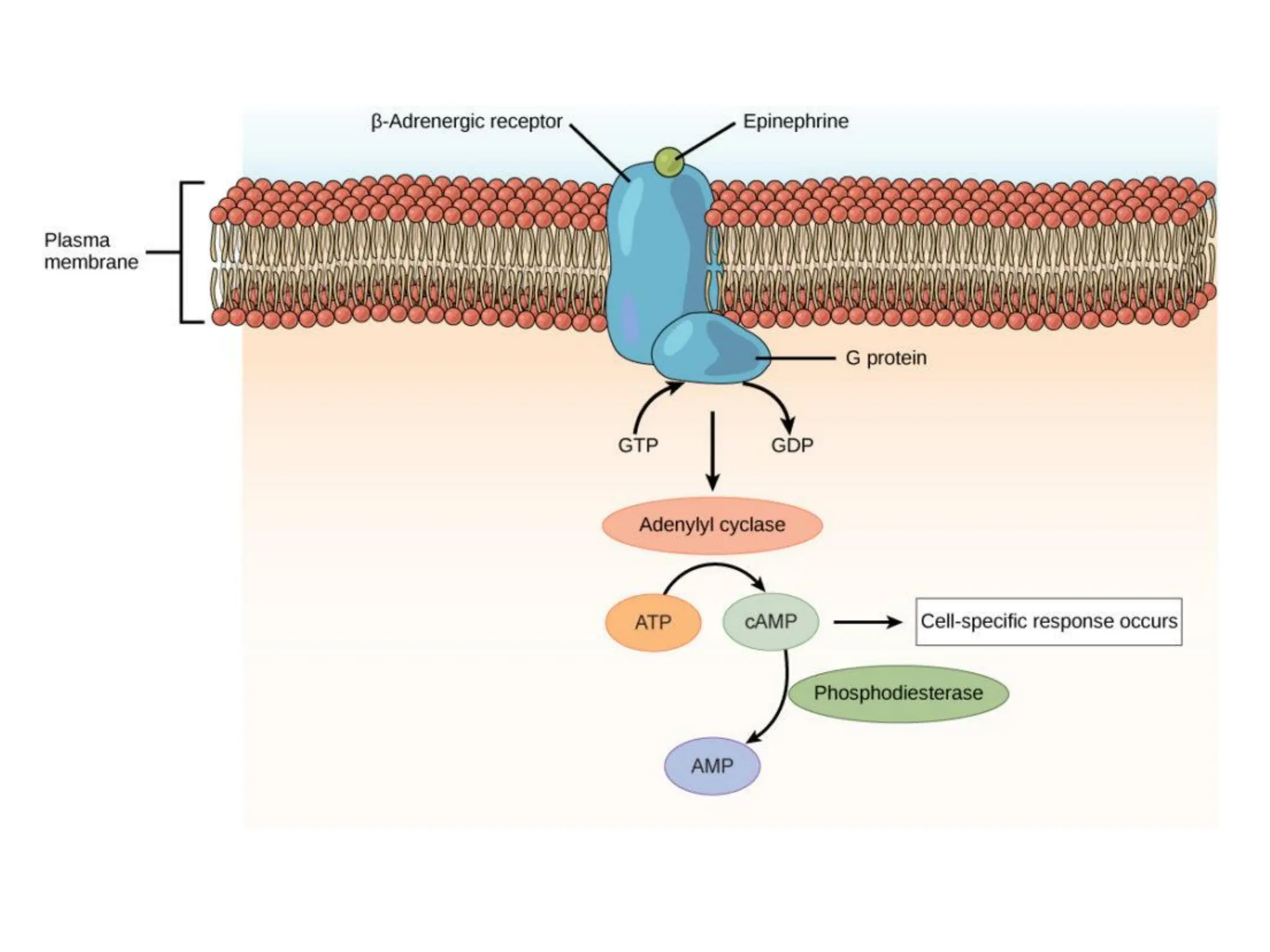
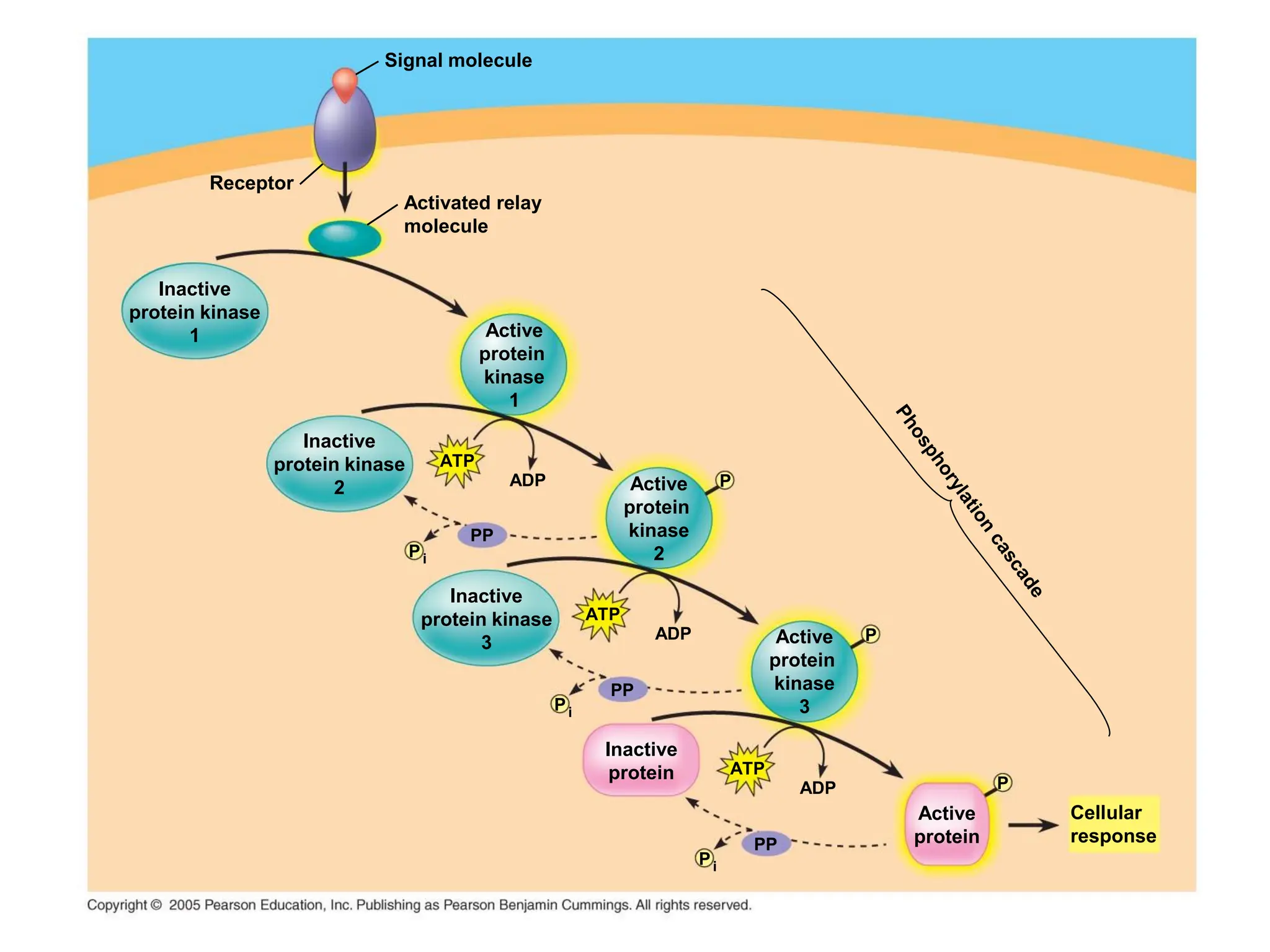
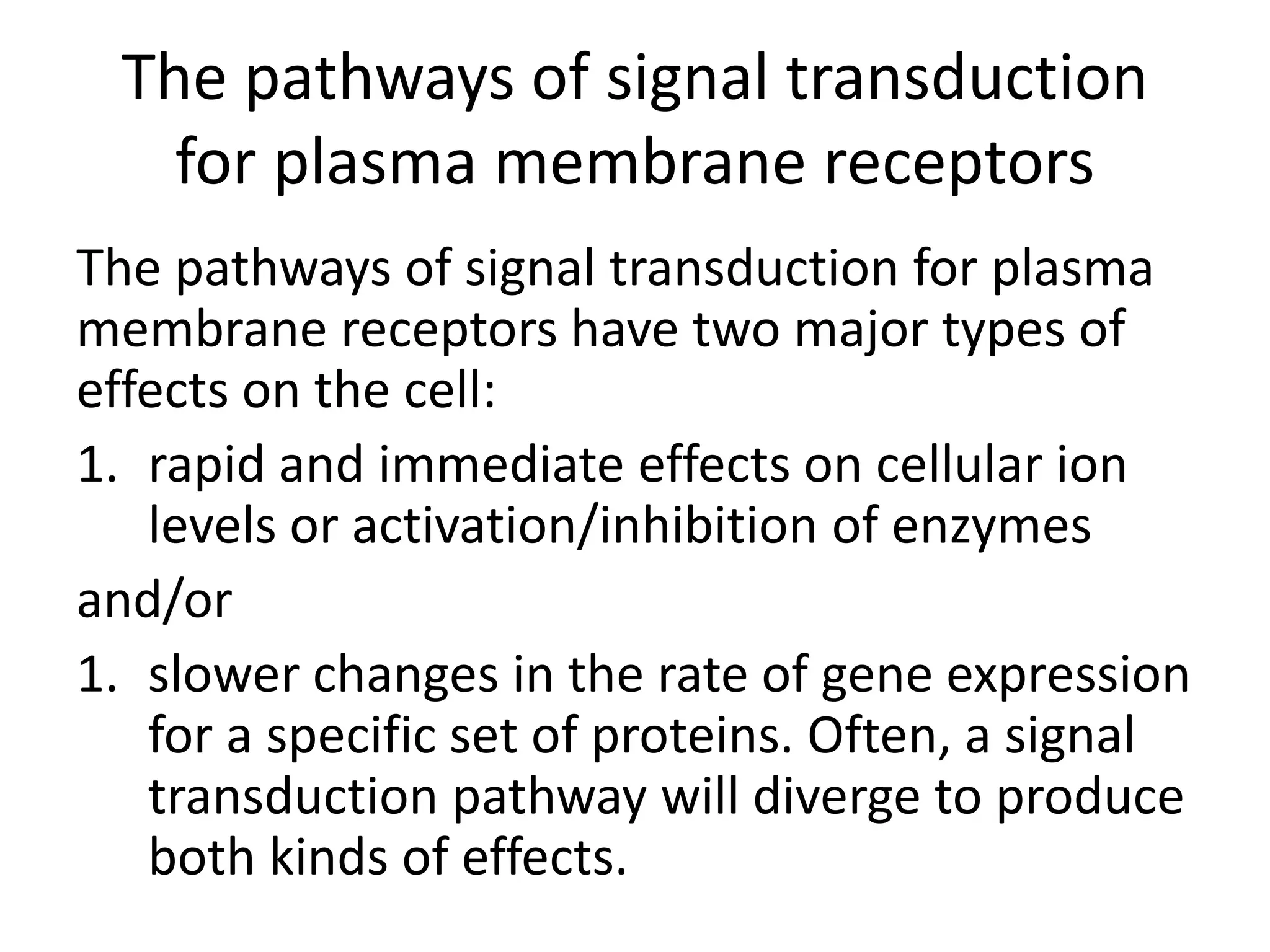
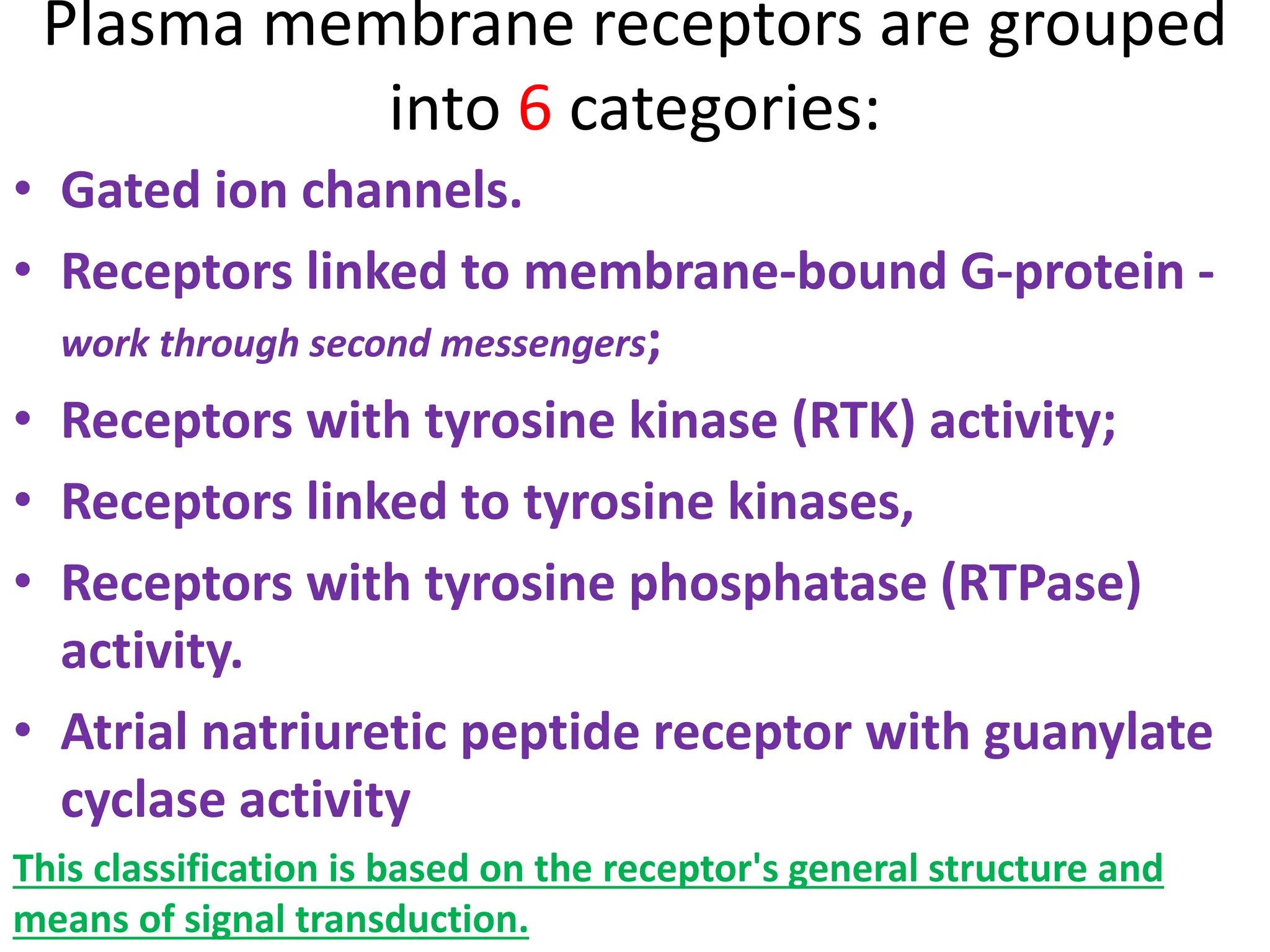
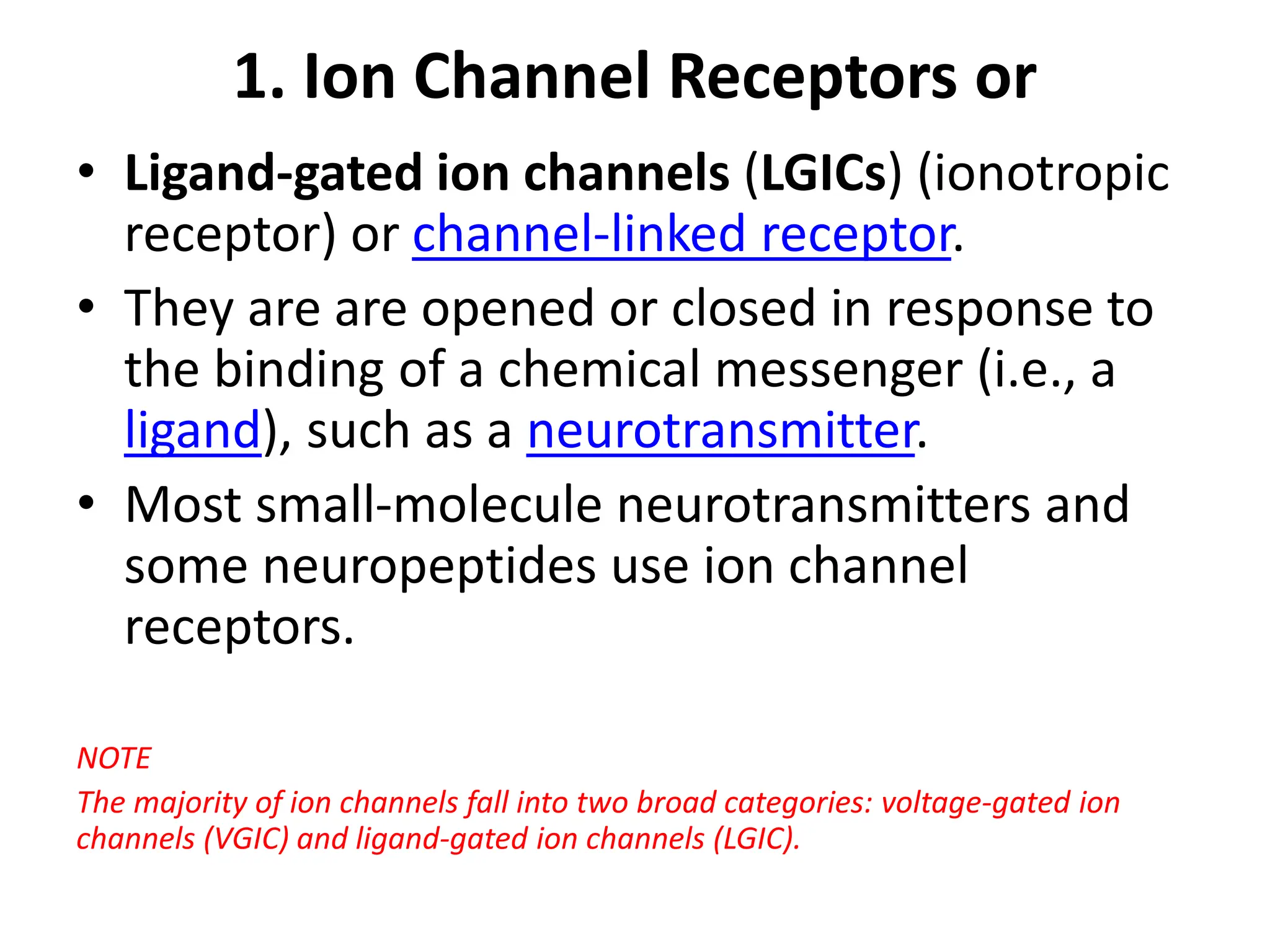
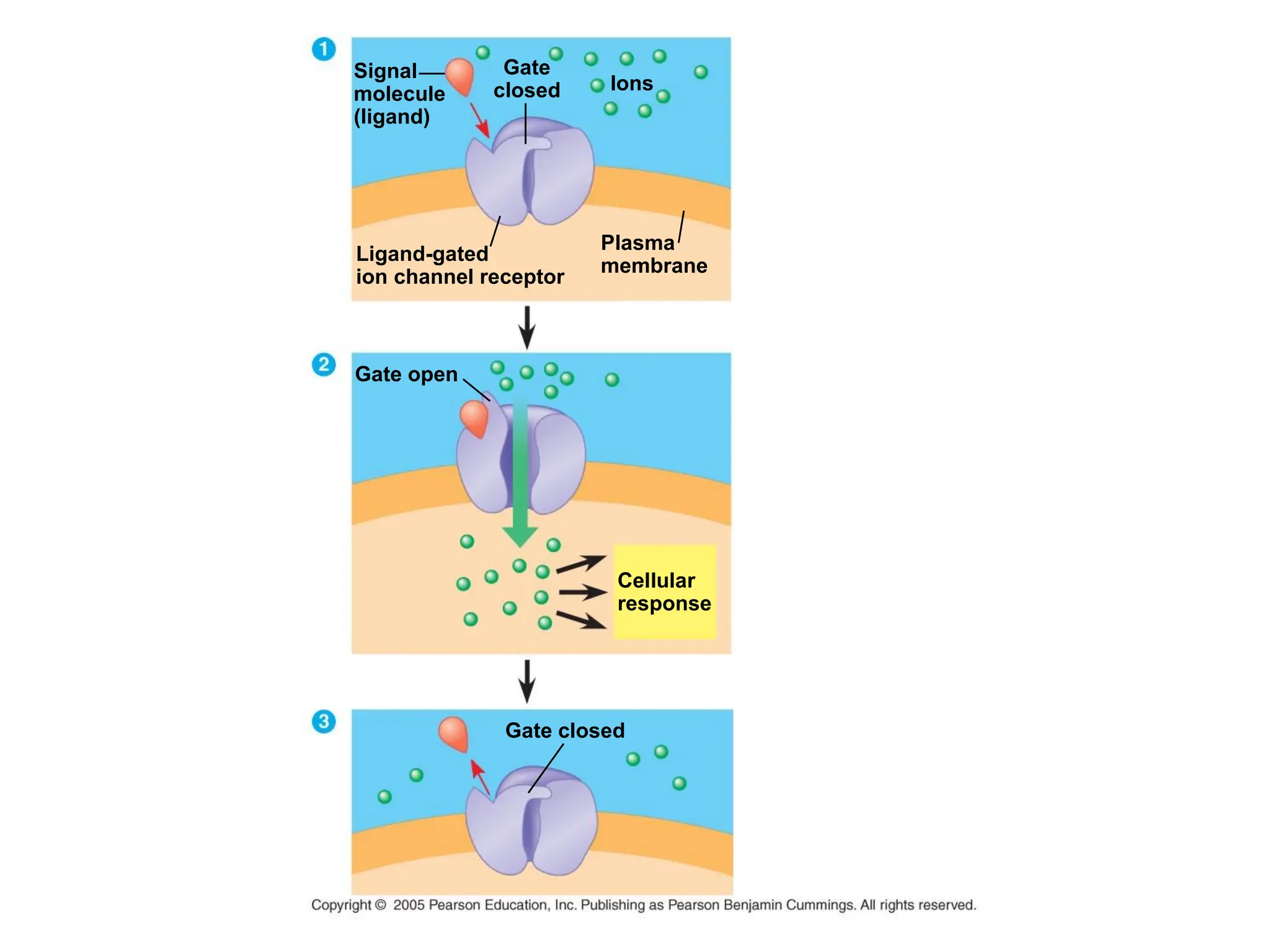
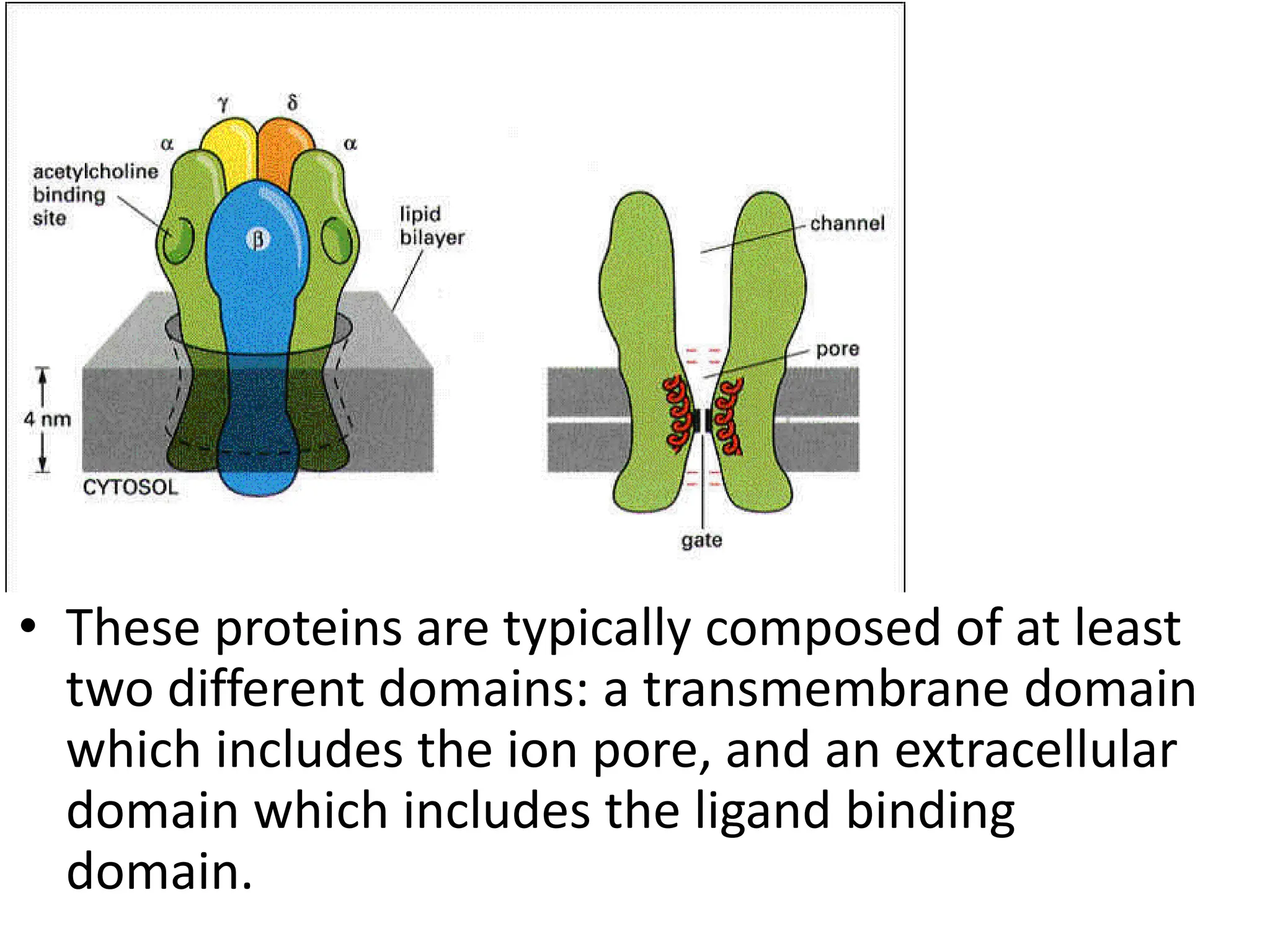
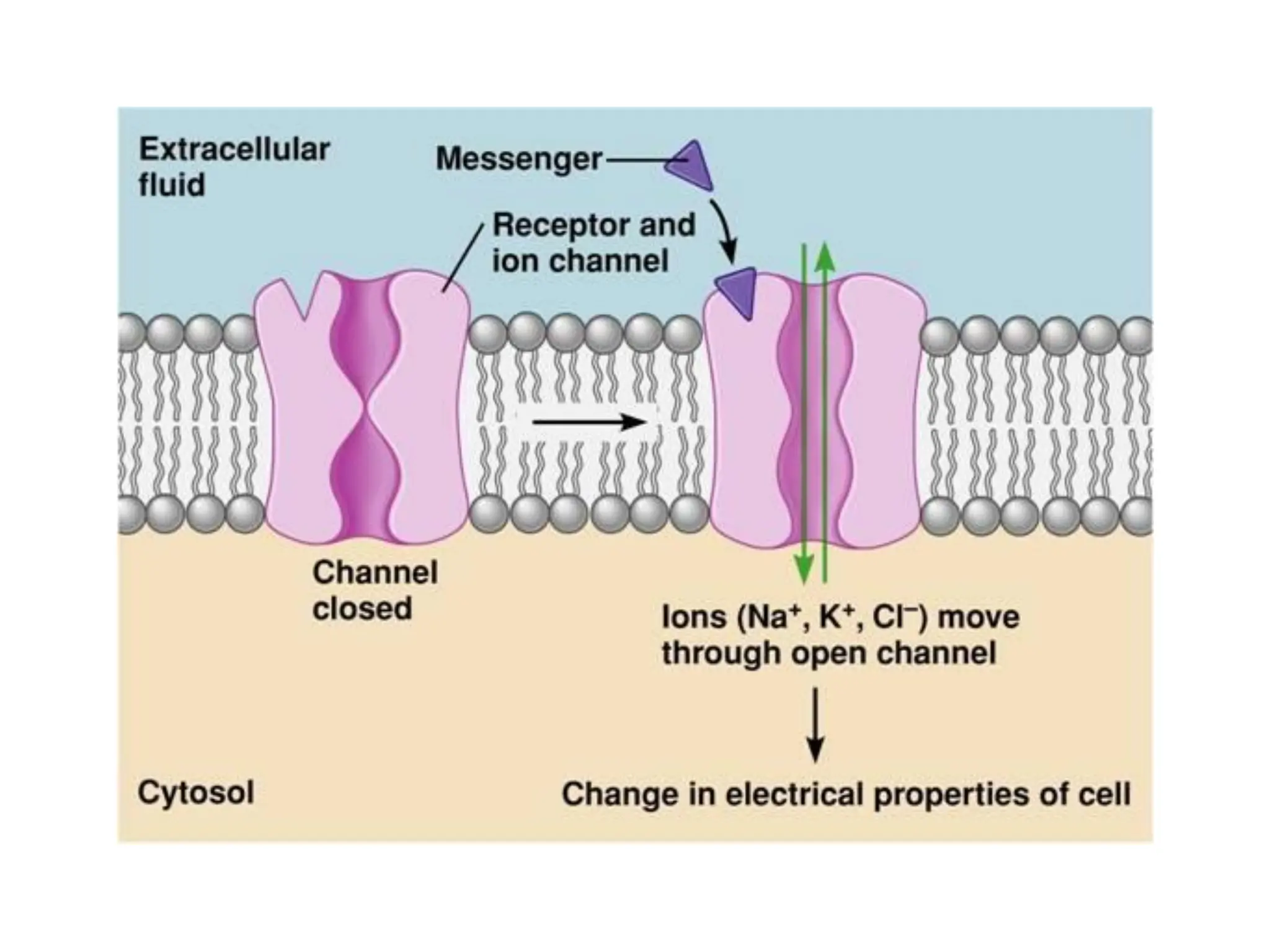
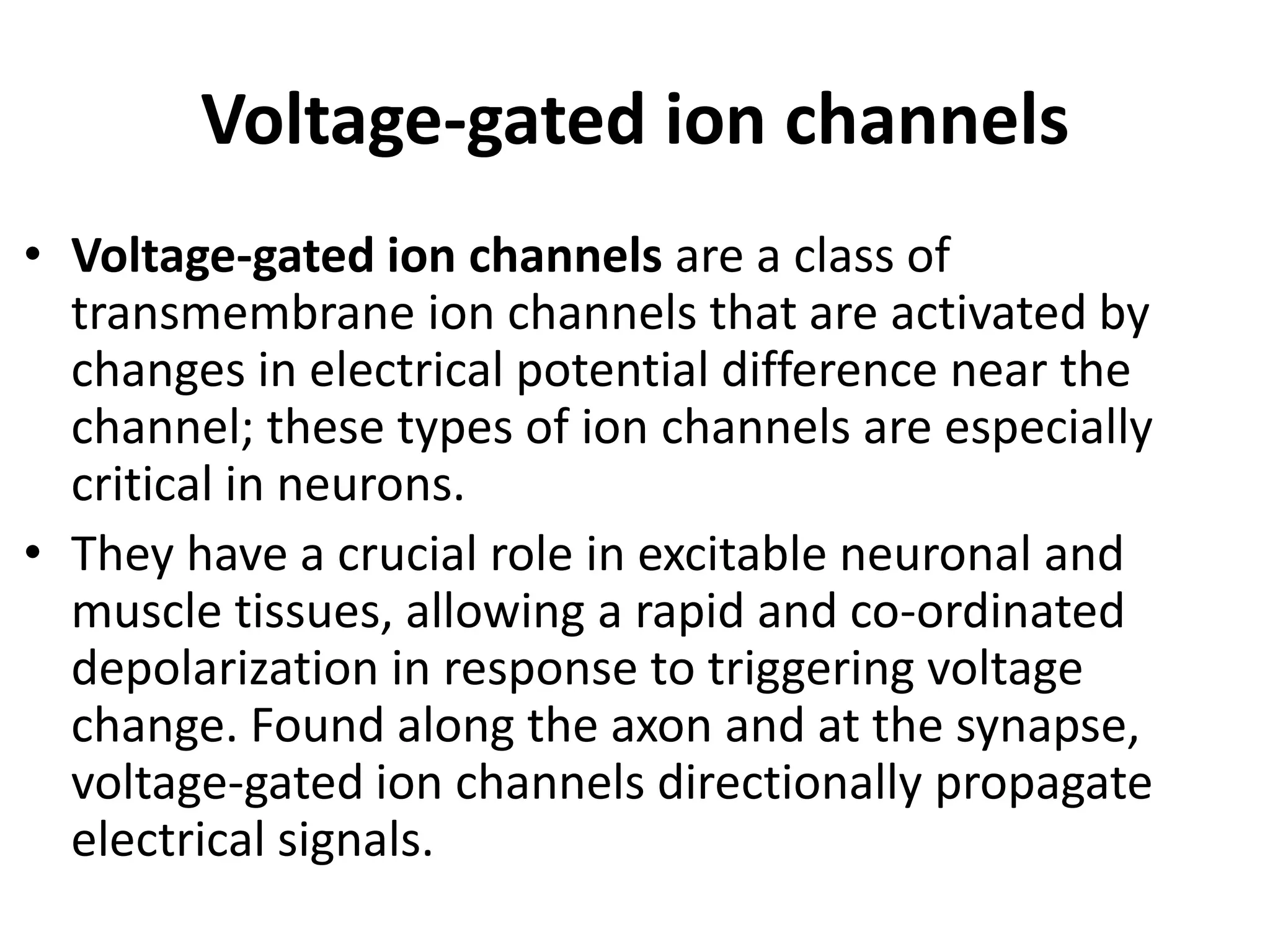
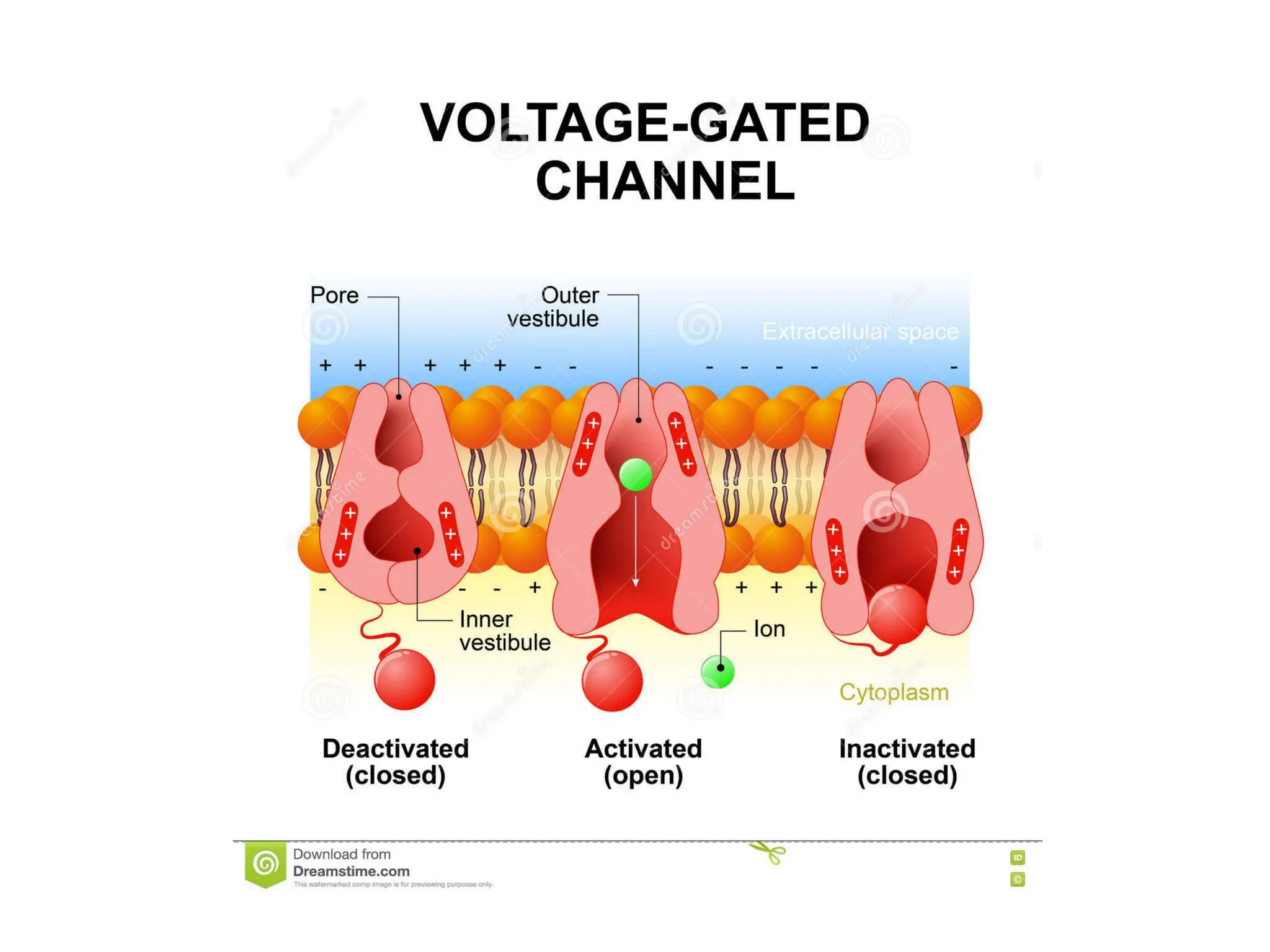
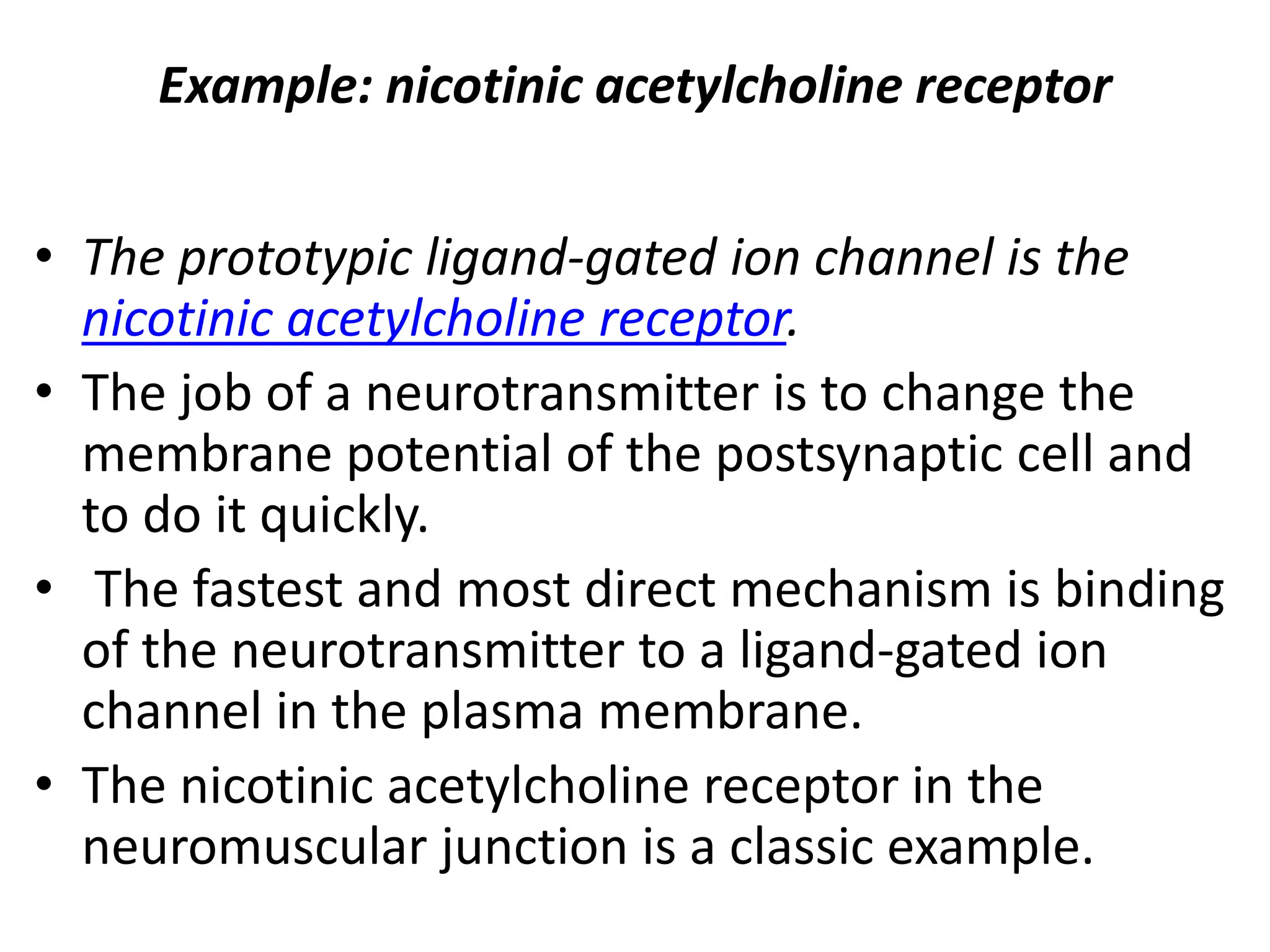
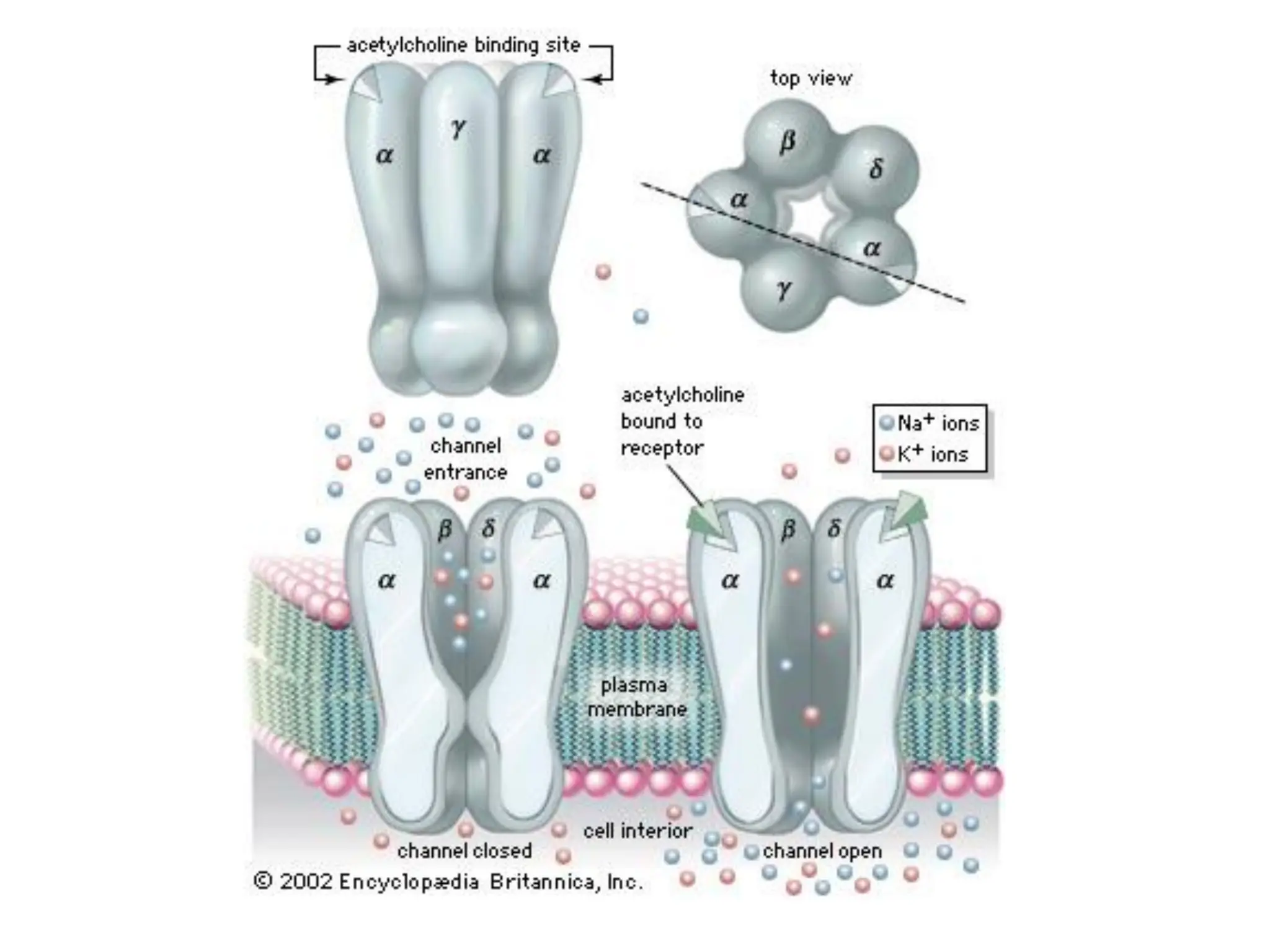
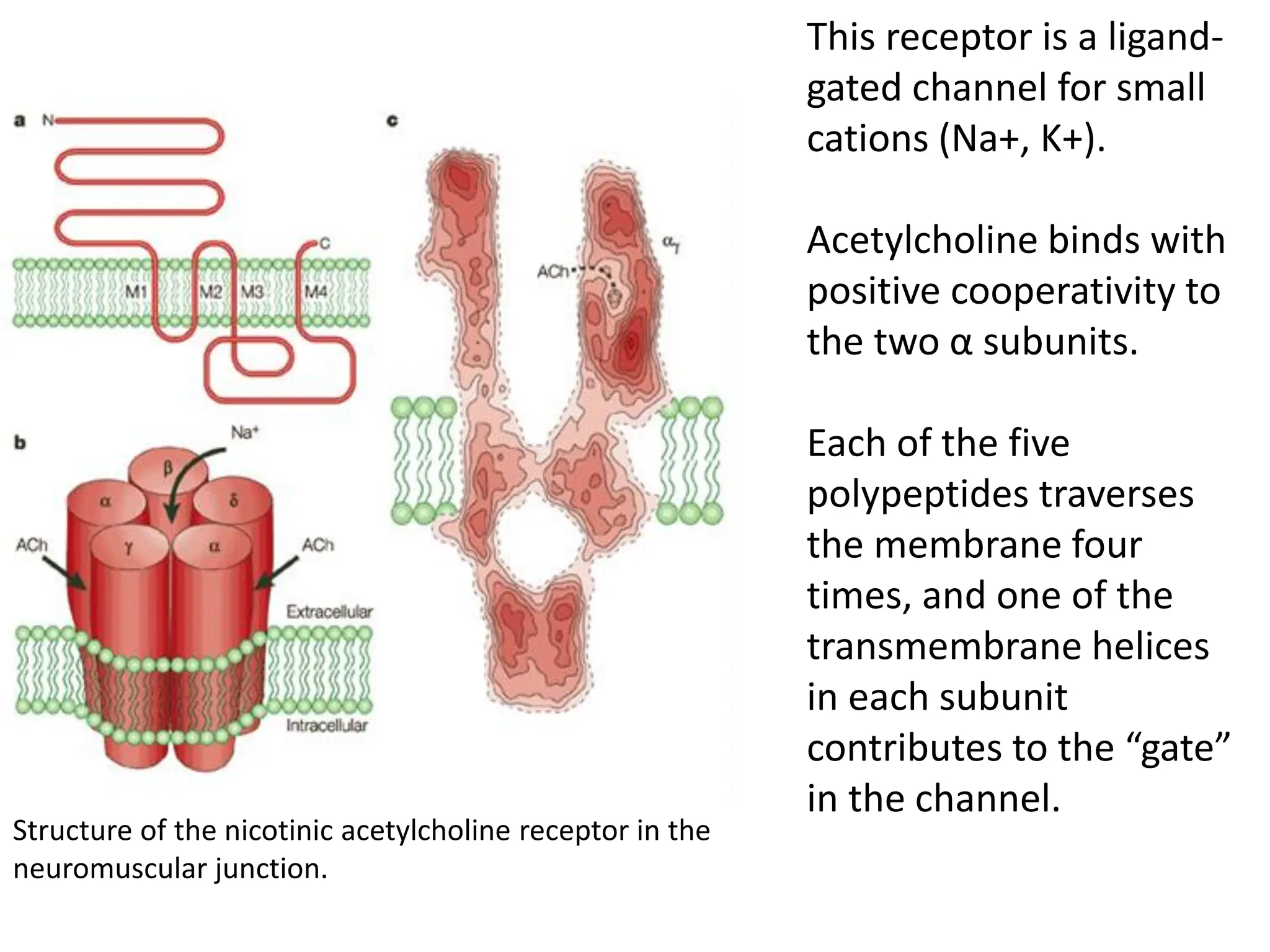
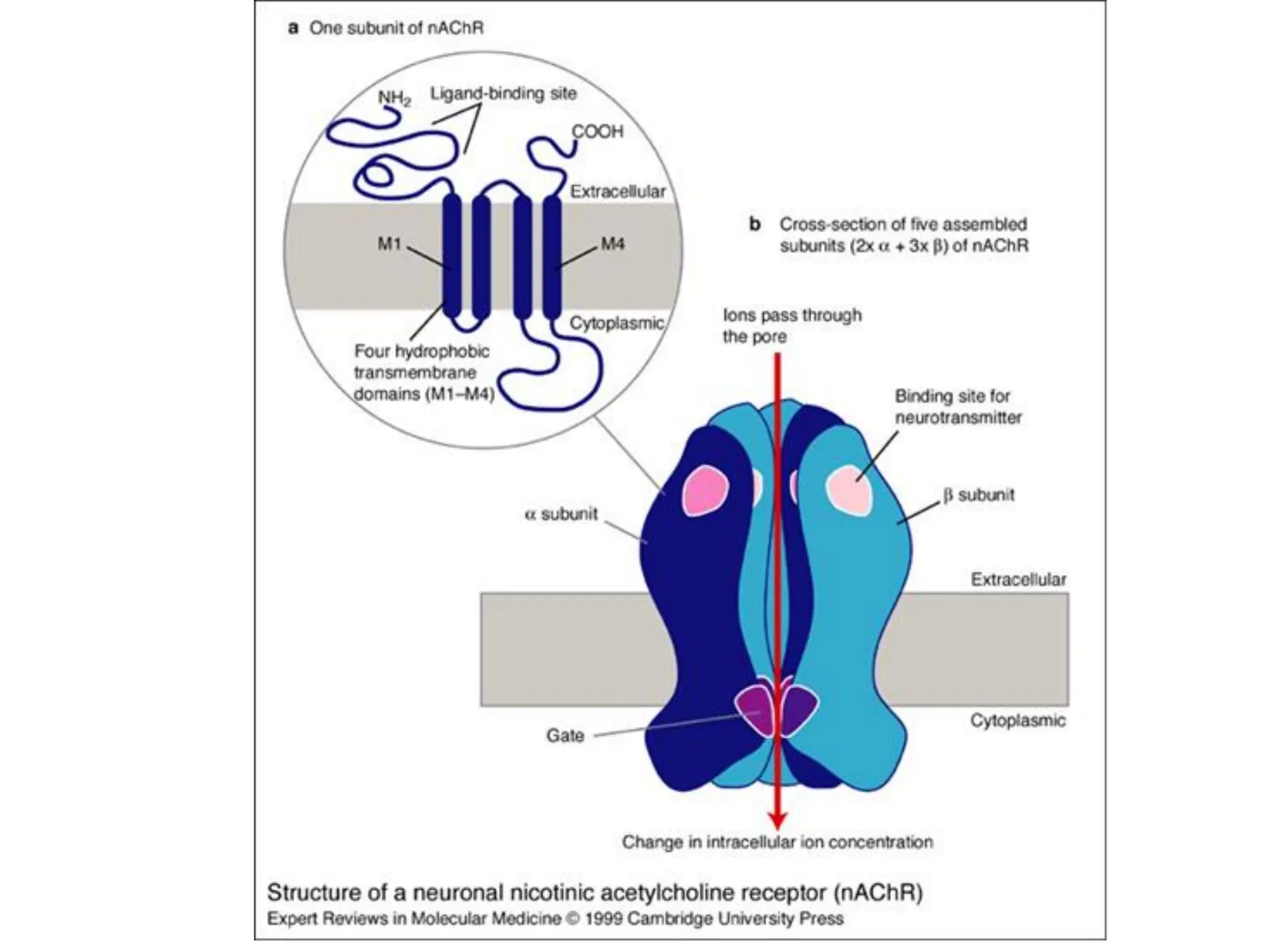
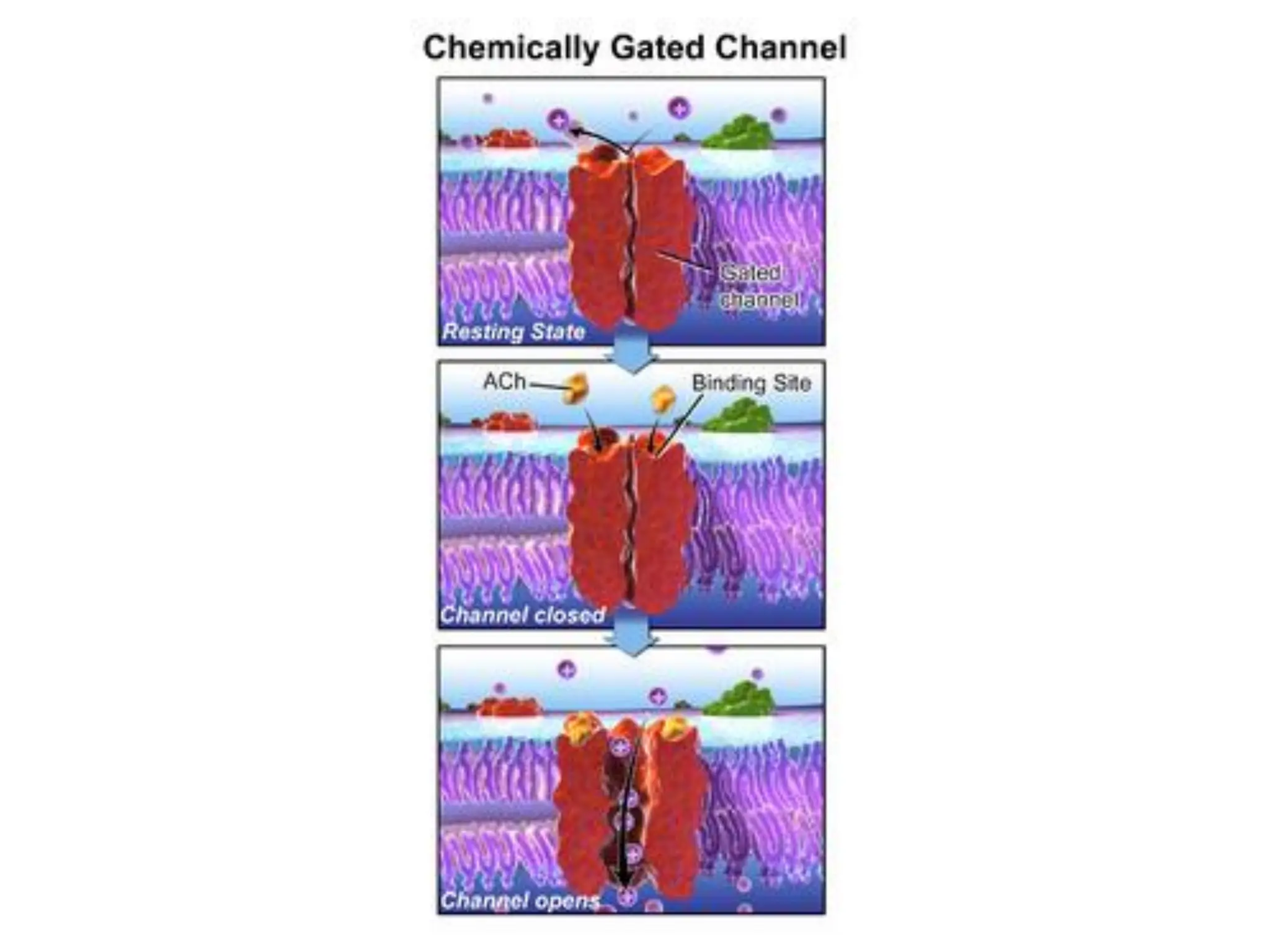
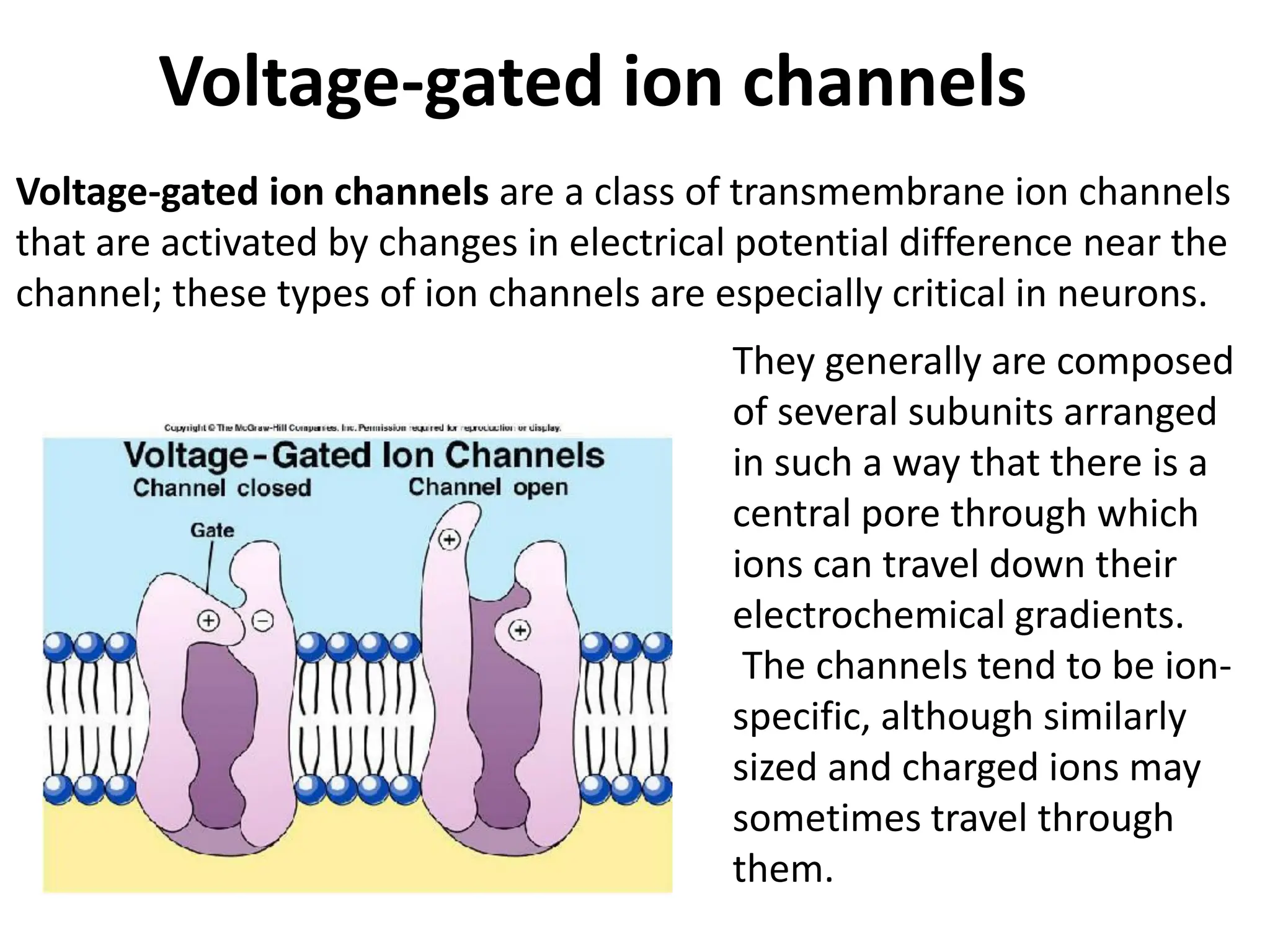
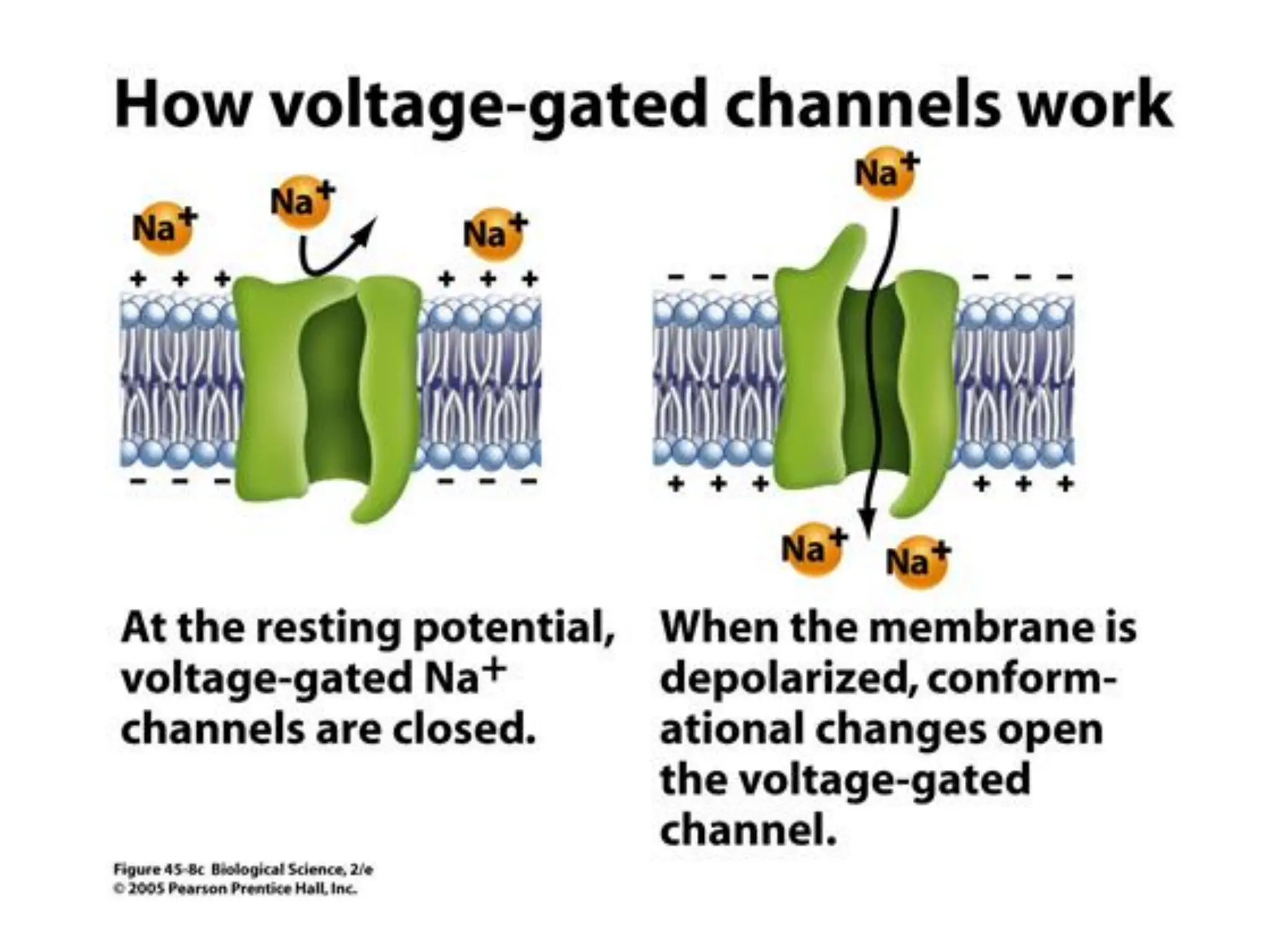
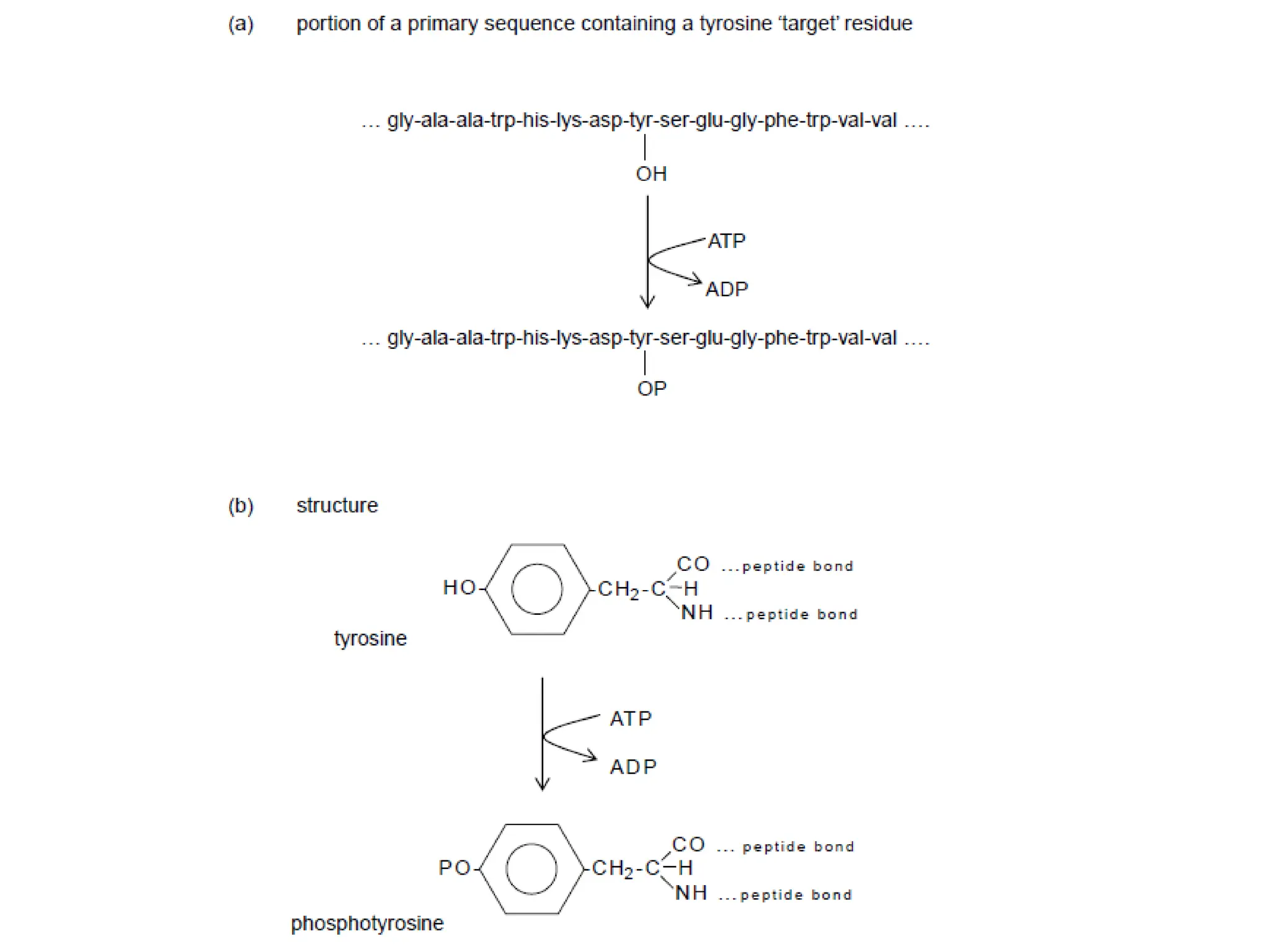
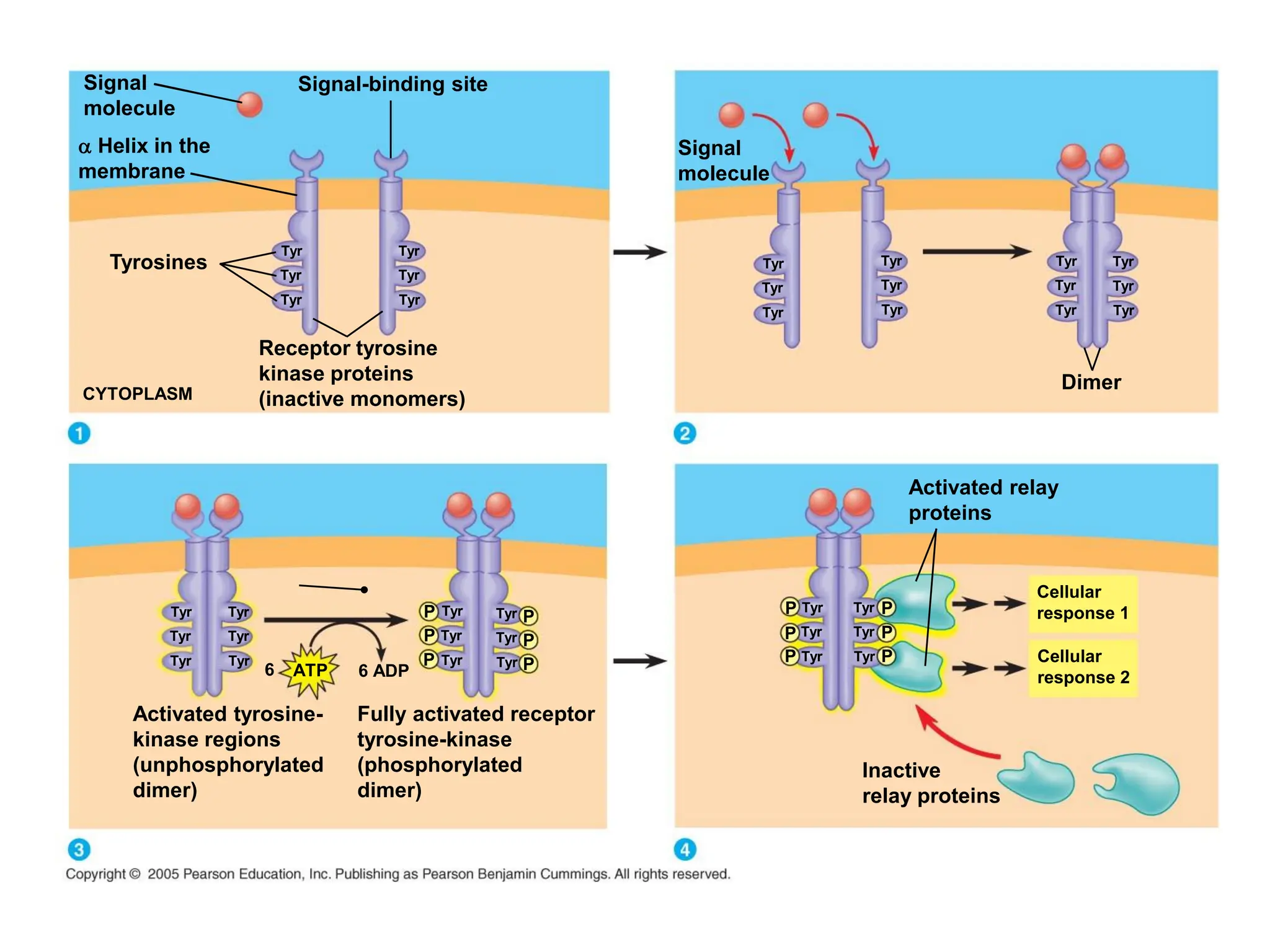
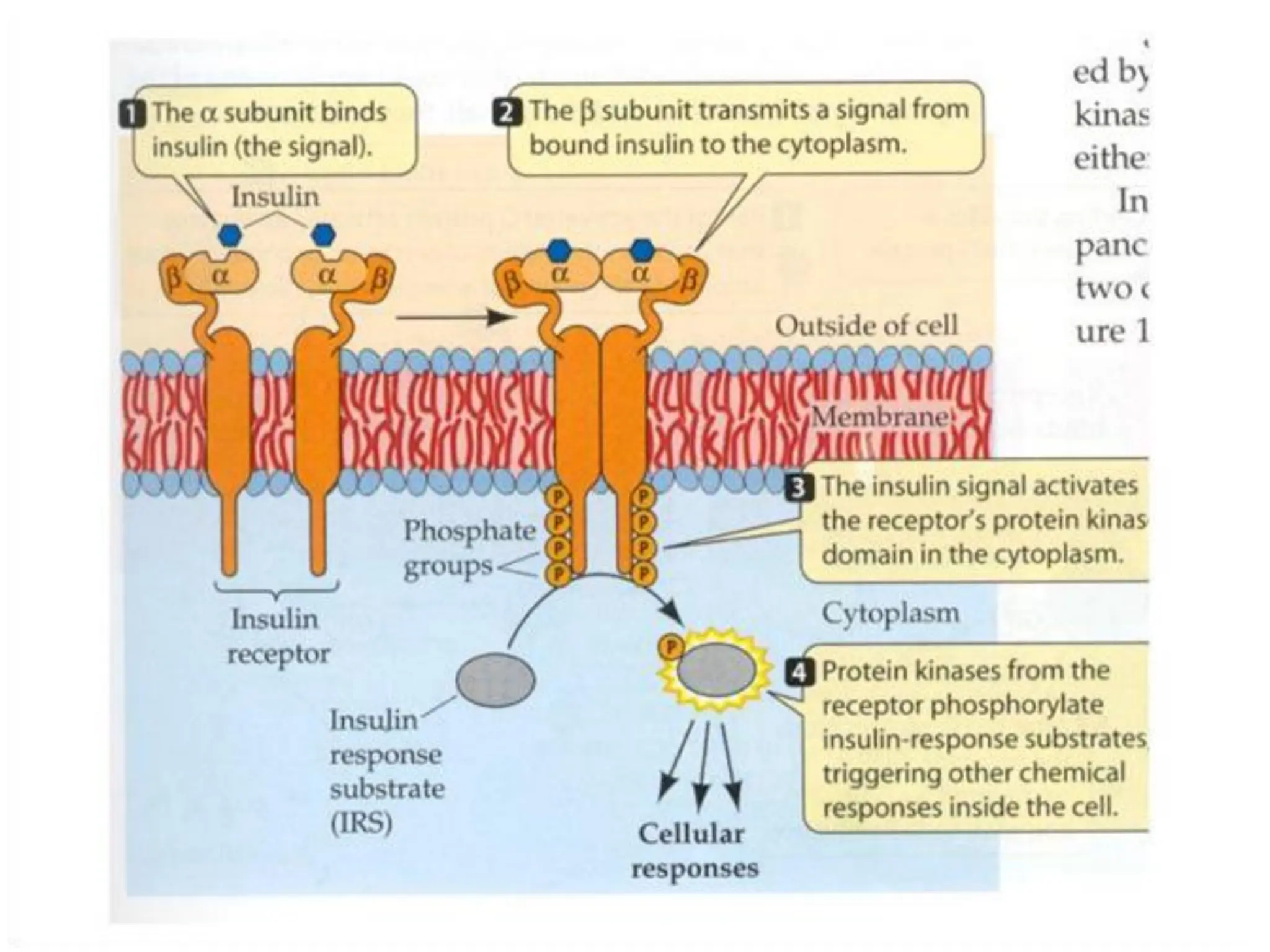
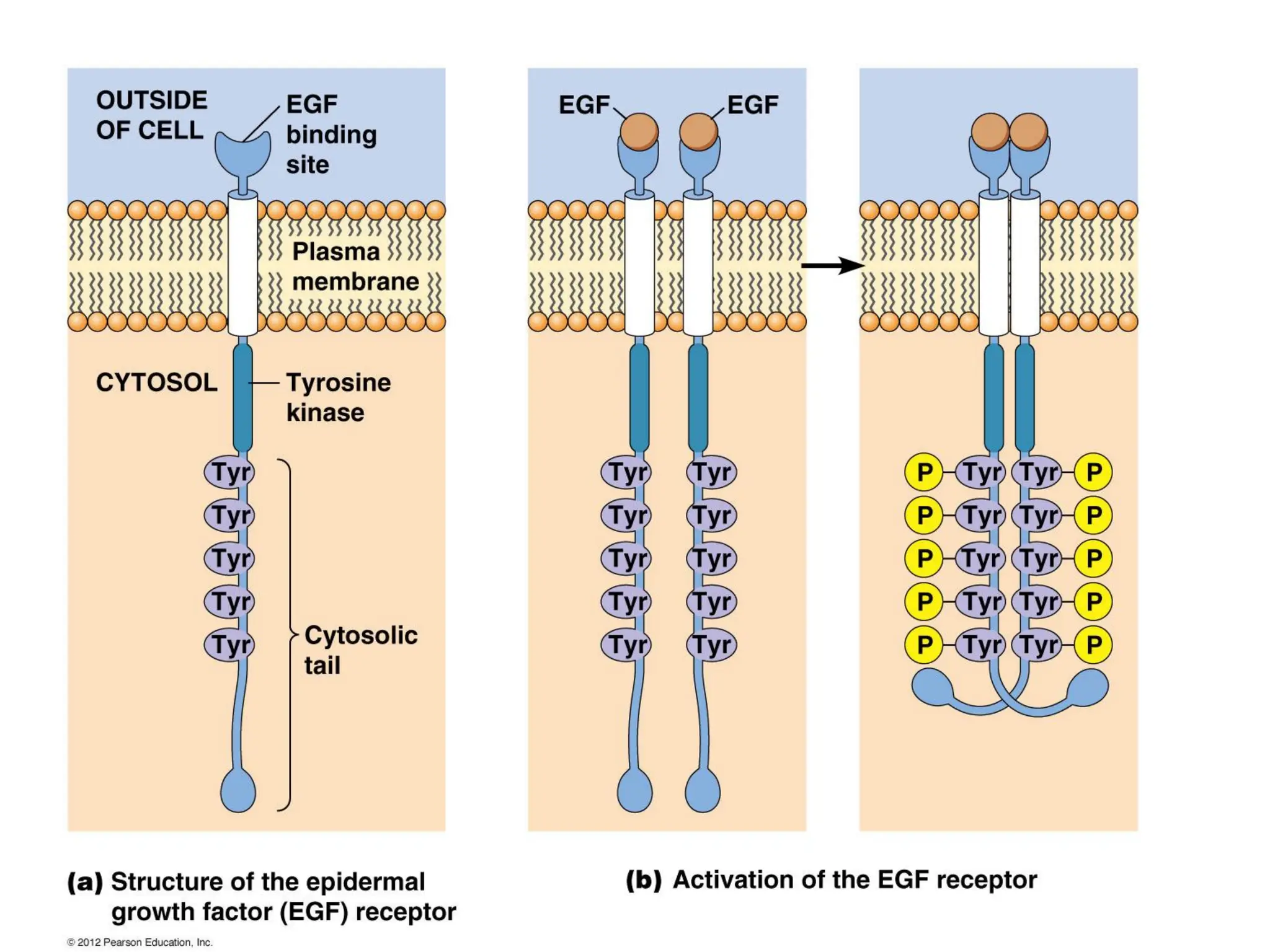
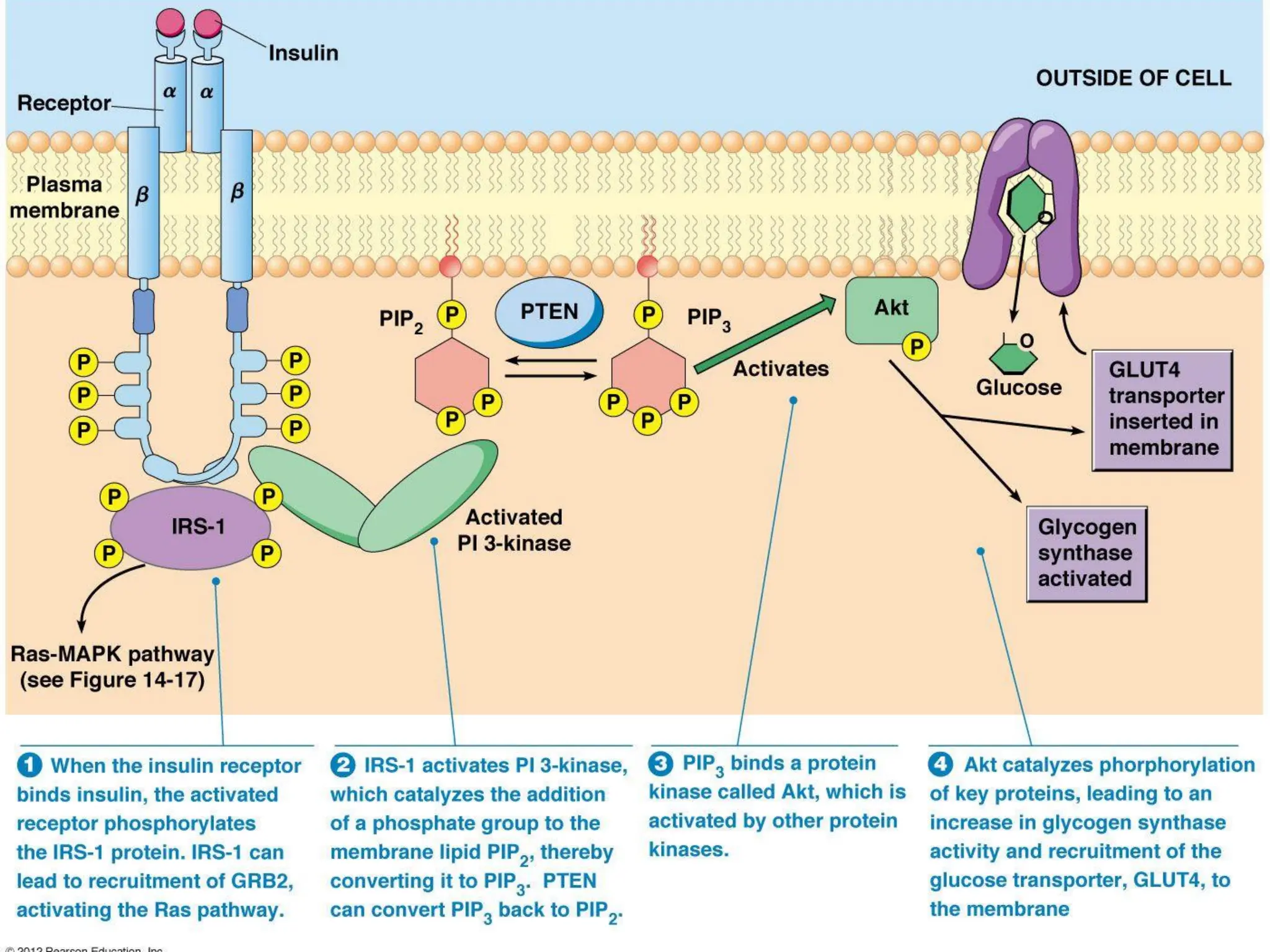
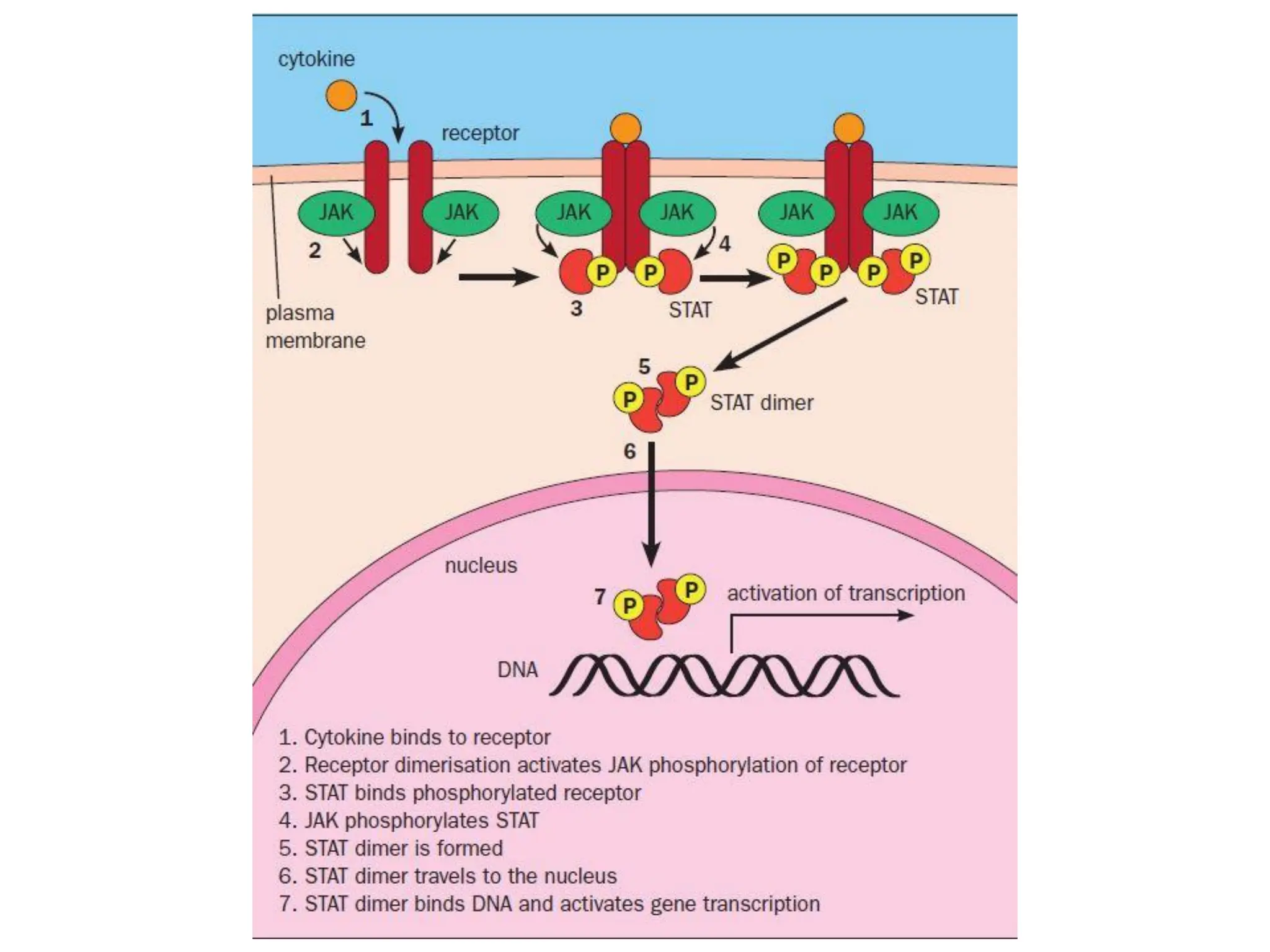
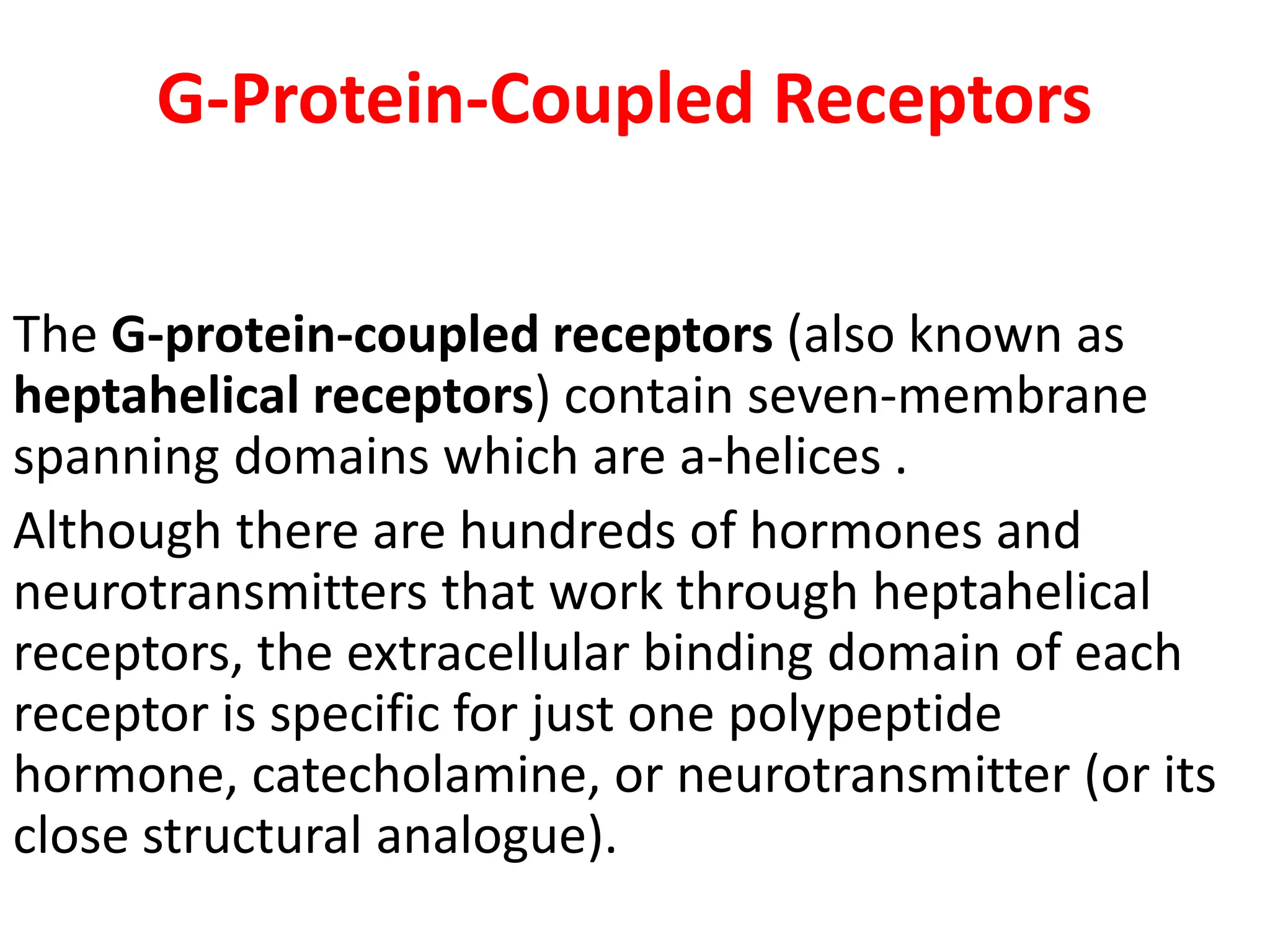
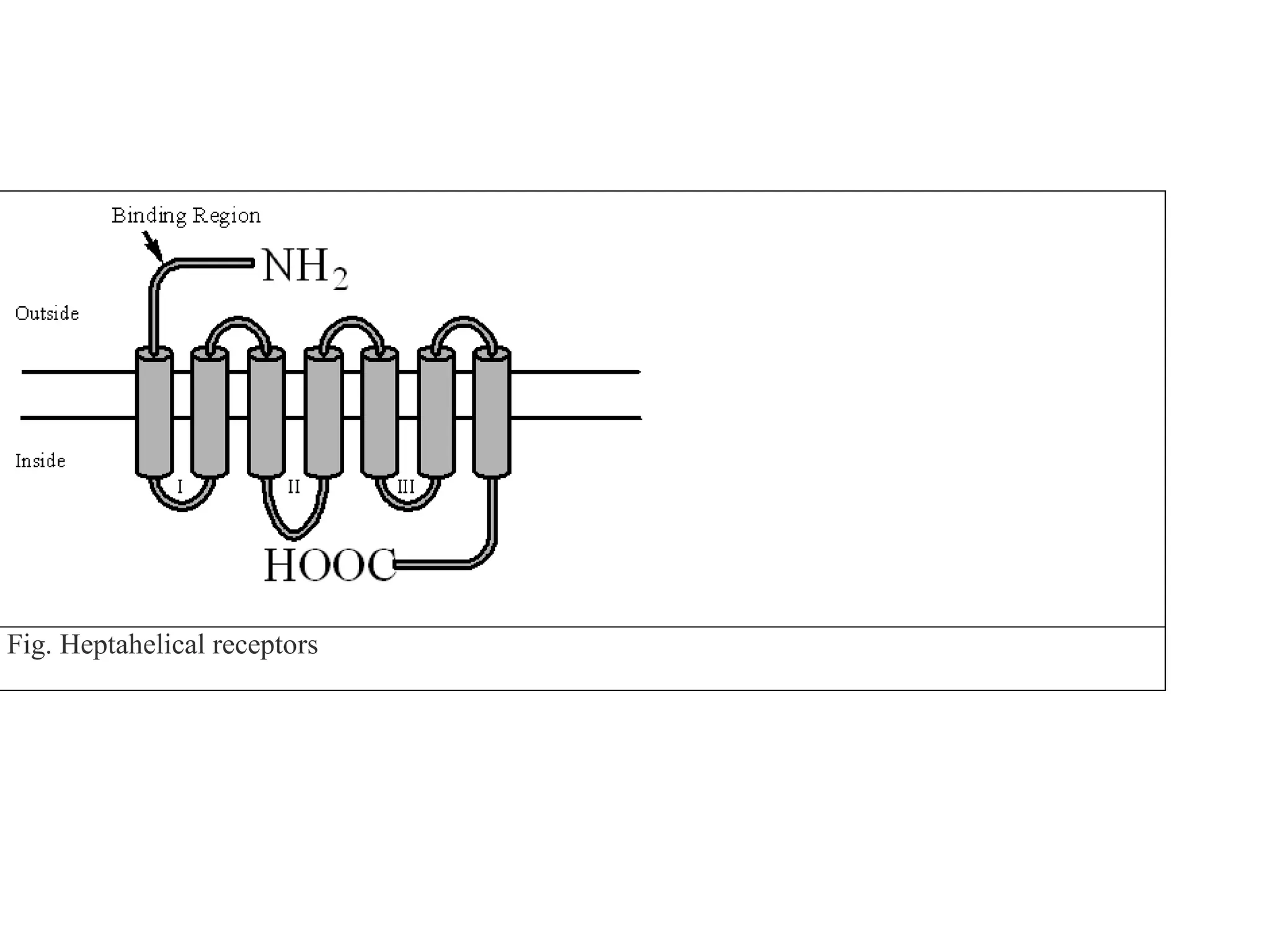
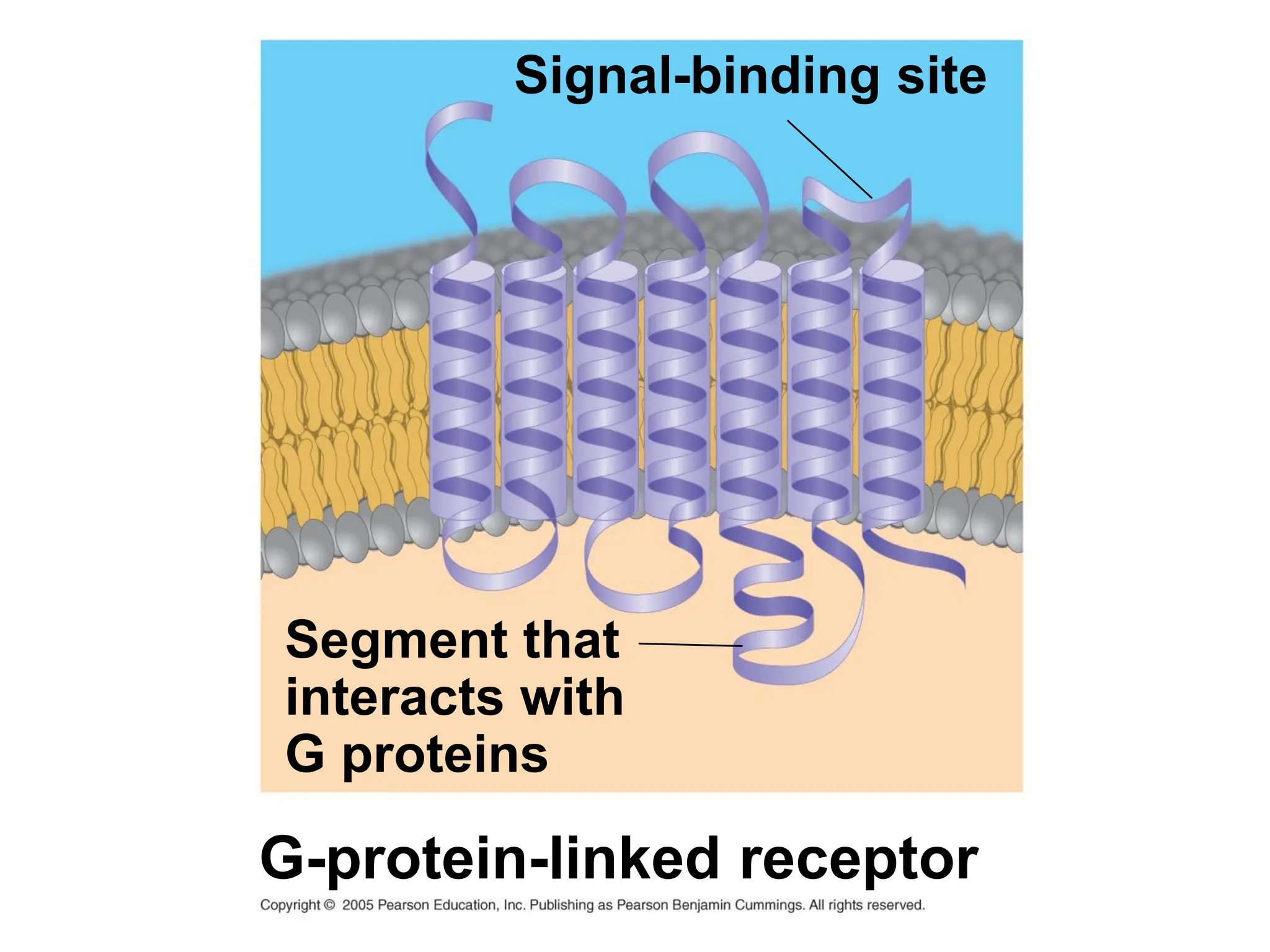
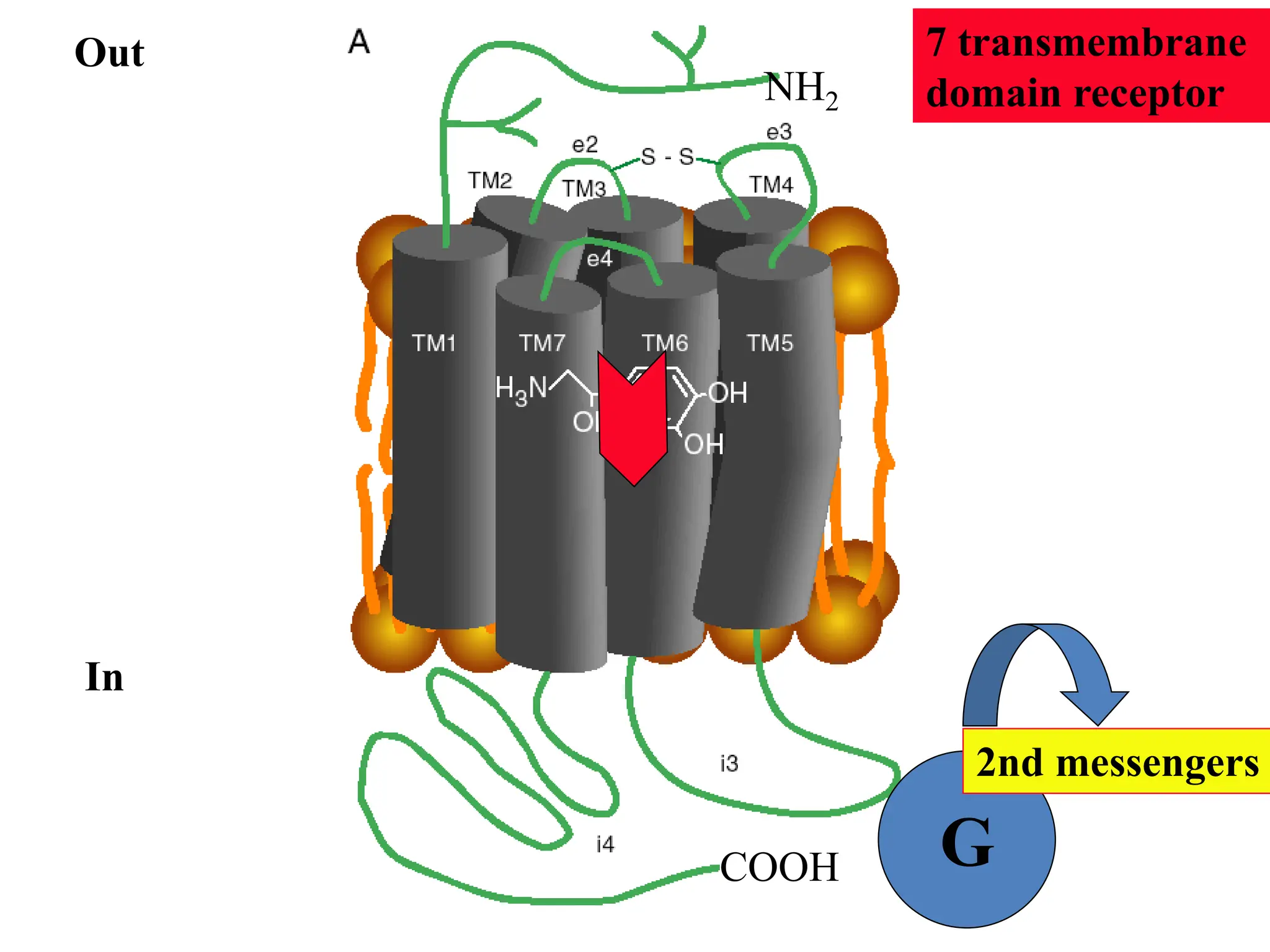
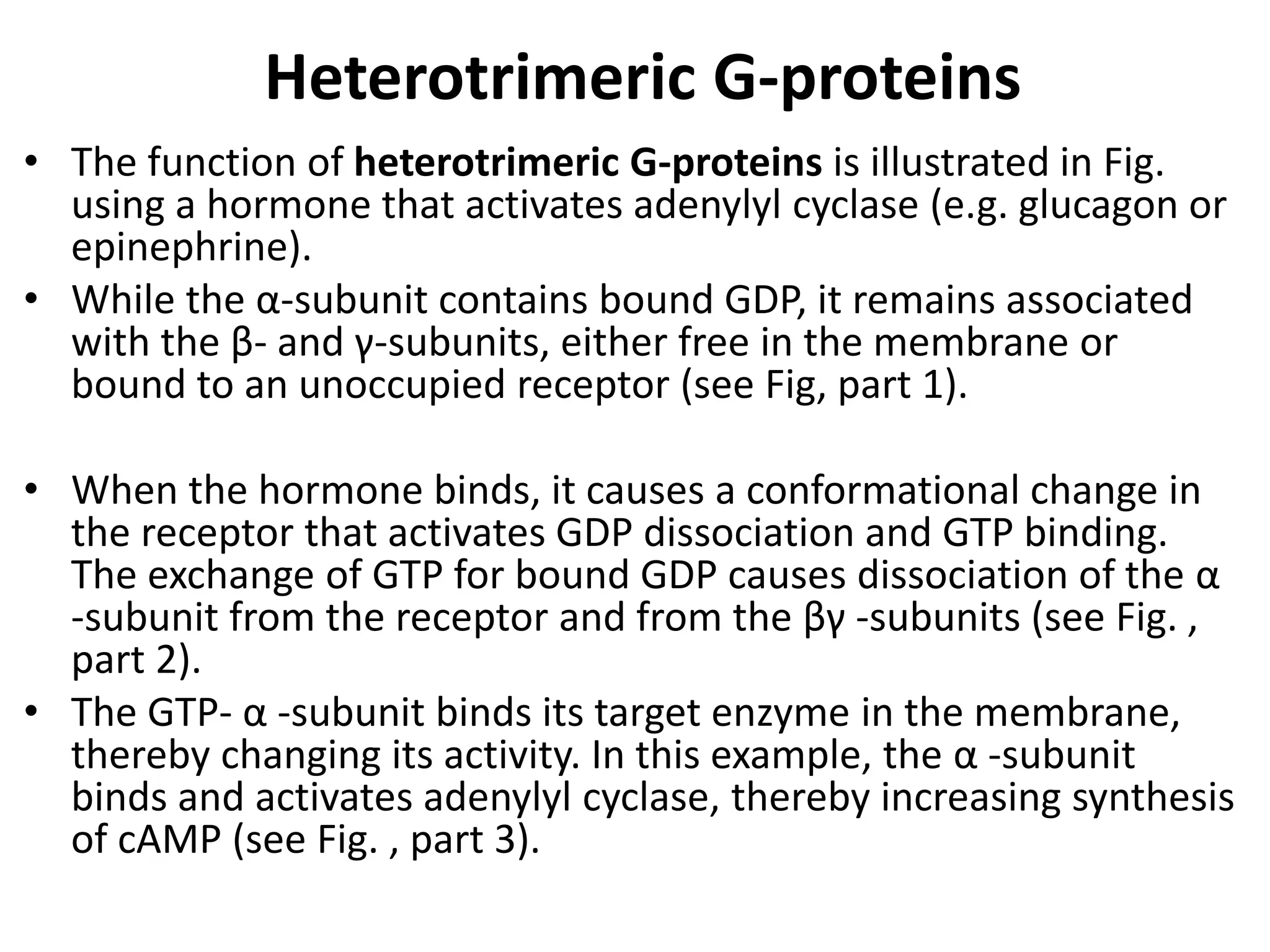
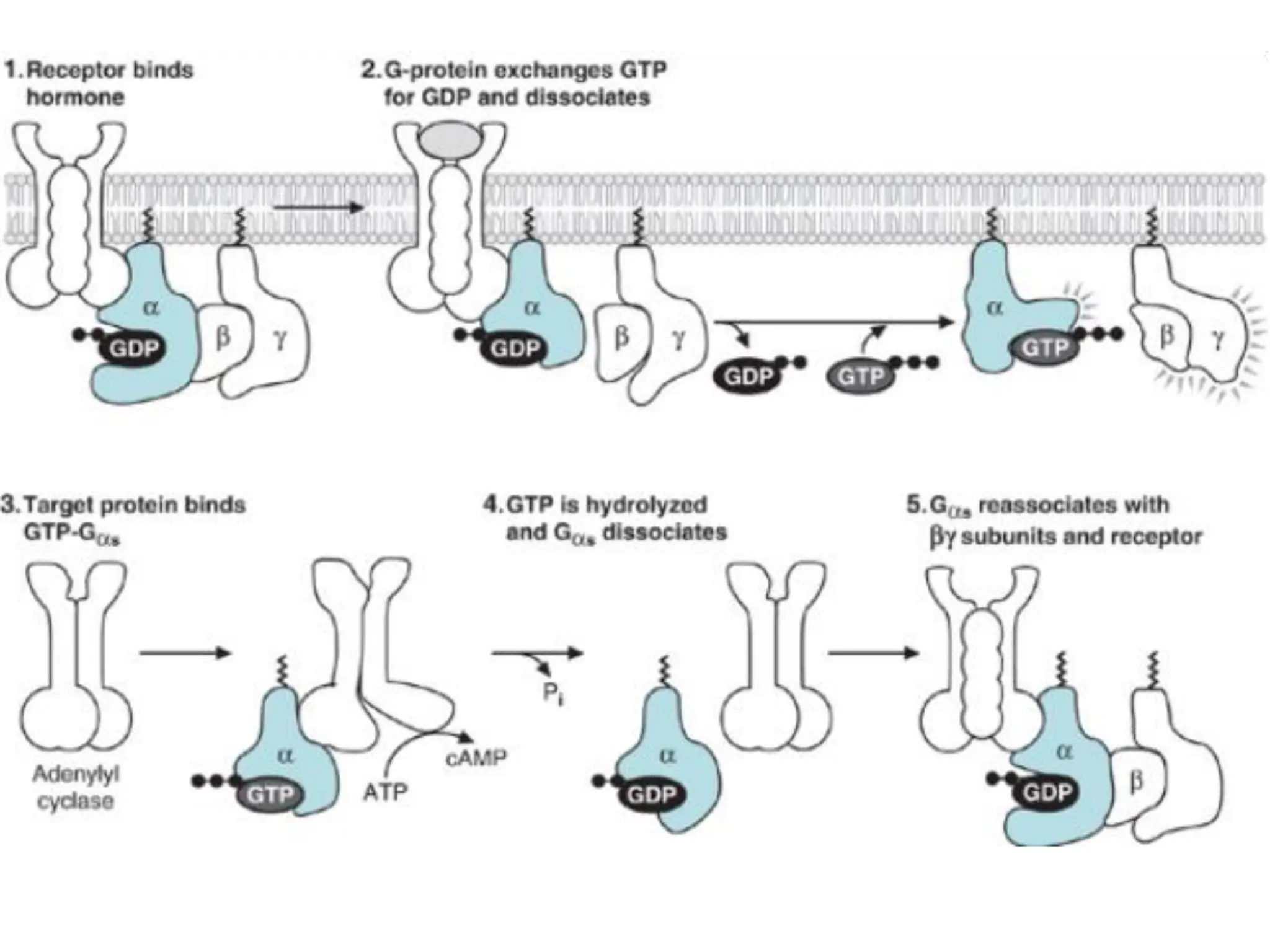
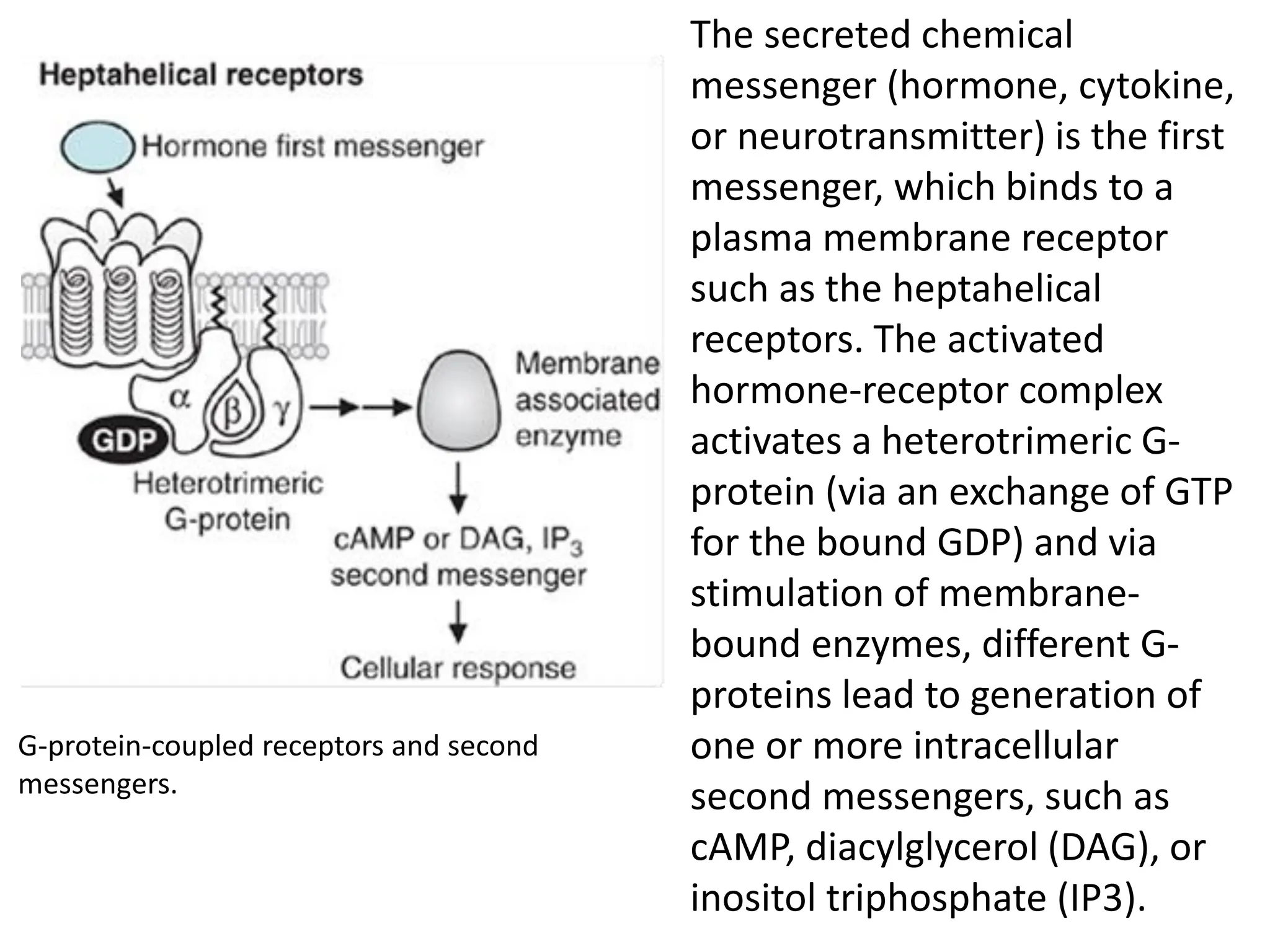
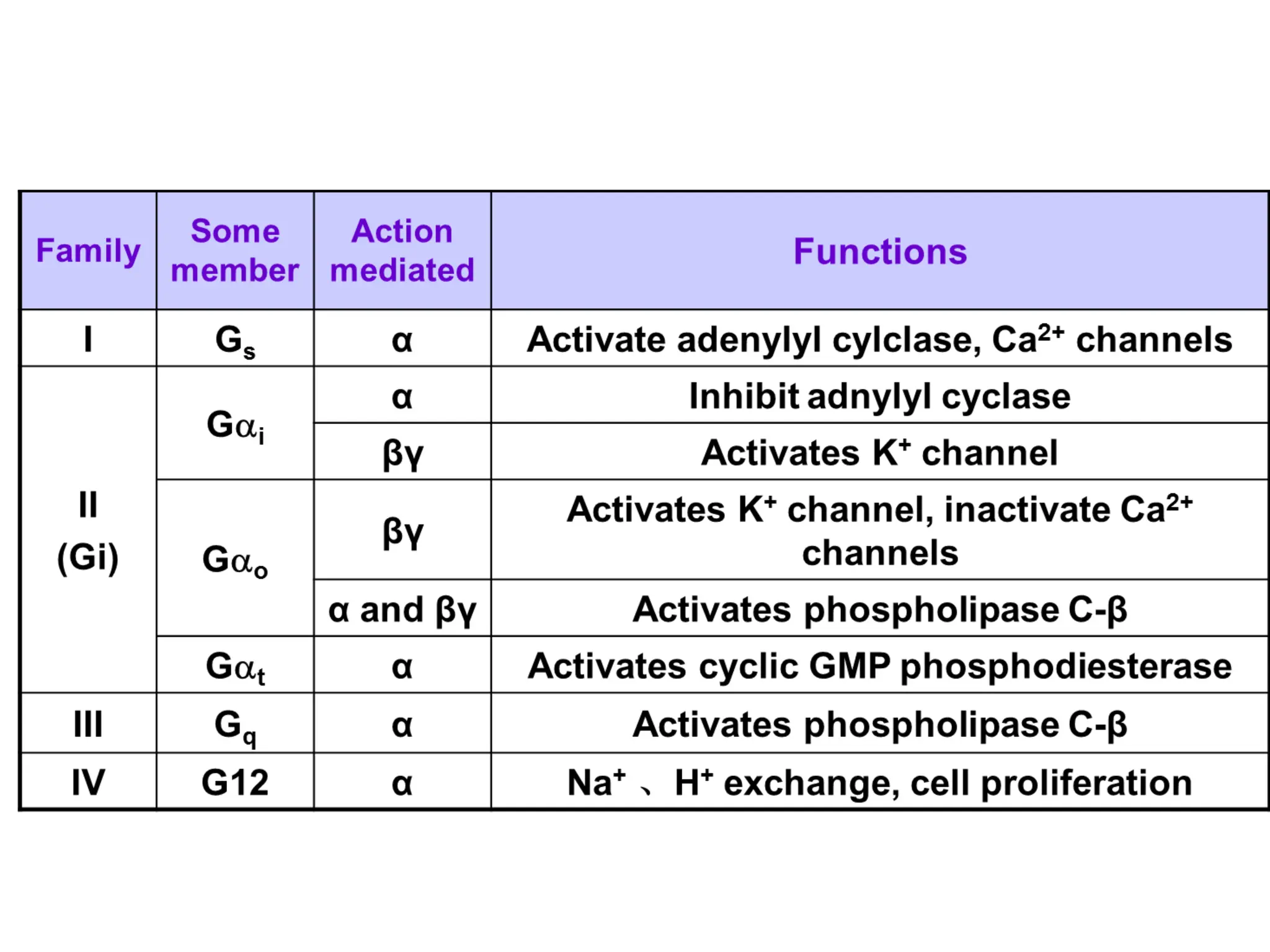
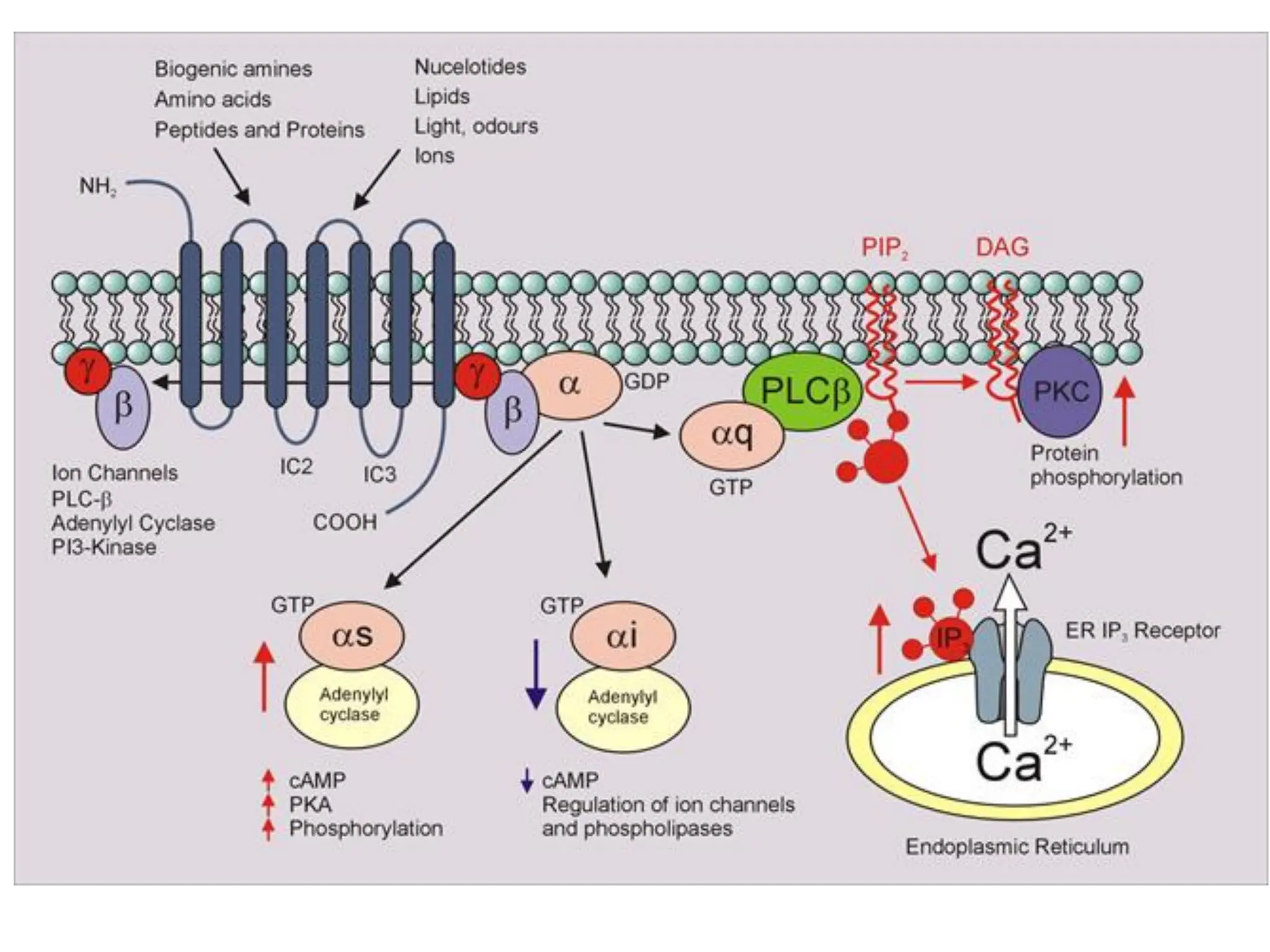
Plasma membrane receptors transmit signals across the cell membrane via several mechanisms of signal transduction. Receptor mechanisms include ligand-gated ion channels that directly open or close in response to ligand binding, initiating electrical signals. Receptor tyrosine kinases dimerize and autophosphorylate upon ligand binding, activating intracellular kinase cascades. G-protein coupled receptors activate heterotrimeric G-proteins upon ligand binding, initiating second messenger signaling pathways. These receptor mechanisms underlie rapid cellular responses or slower changes in gene expression.