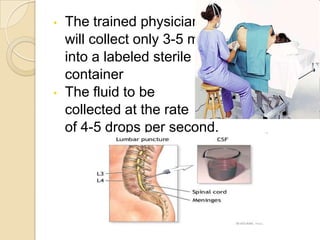
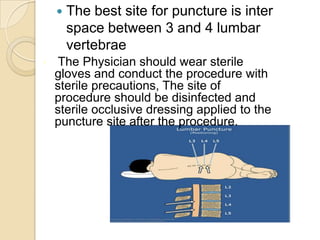
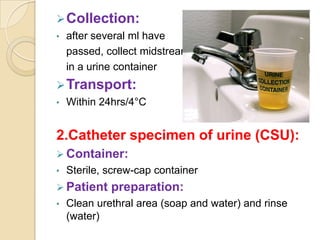
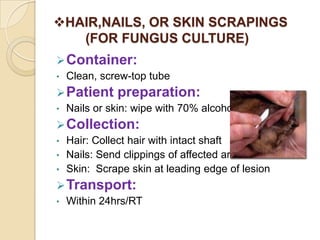
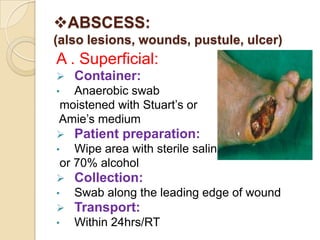
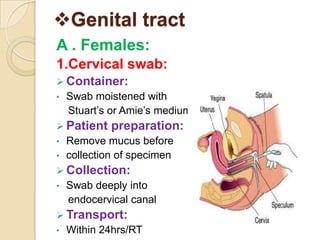
1. Proper specimen collection and transport are critical for laboratory testing.
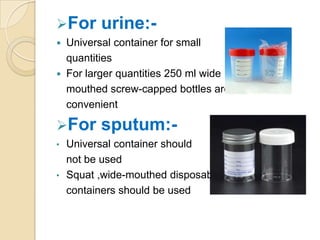
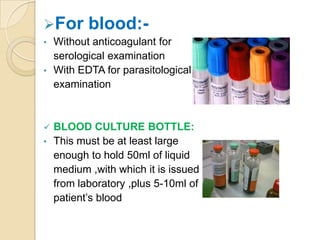
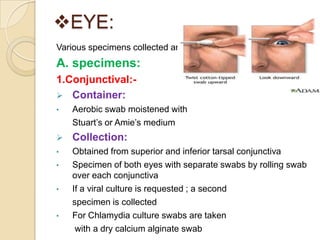
2. Guidelines for collection include using appropriate containers and transport media, minimizing contamination, and providing complete patient information.
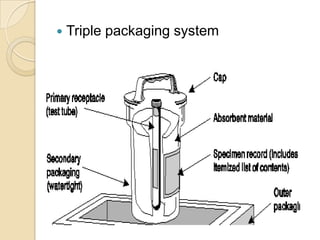
3. Specimens should be transported within 2 hours of collection and delivered to the laboratory promptly using biohazard labeling and packaging.