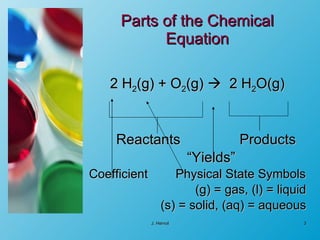
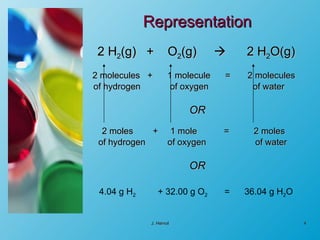
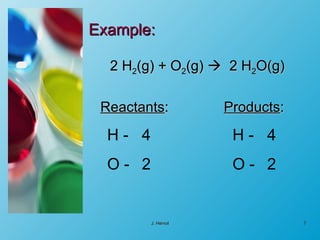
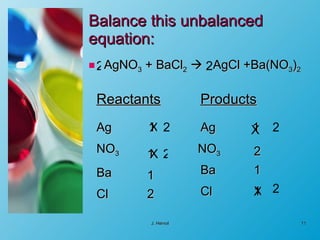
The chemical equation is a written representation of a chemical reaction showing the reactants and products using chemical formulas and symbols. It must obey the laws of conservation of mass and matter, meaning the same number and type of atoms are on both sides and the total mass is conserved. Examples are provided to demonstrate balancing chemical equations so the number of each type of atom is equal on both sides.