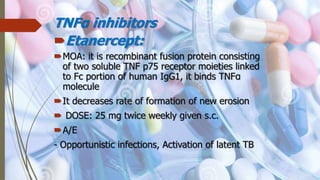
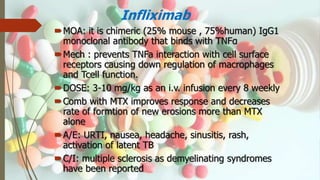
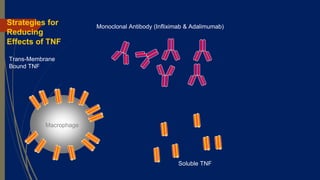
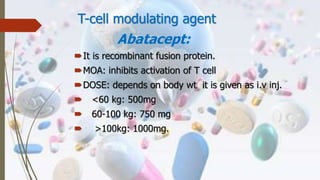
This document discusses disease modifying anti-rheumatic drugs (DMARDs) used to treat rheumatoid arthritis. It describes two main categories of DMARDs - synthetic DMARDs like methotrexate, sulfasalazine, and hydroxychloroquine, and biological DMARDs that target specific proteins in the immune system like TNF inhibitors etanercept and infliximab. It provides details on the mechanisms of action, dosages, and side effects of various DMARDs commonly used as first-line and subsequent options for treating rheumatoid arthritis.















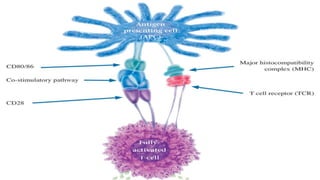
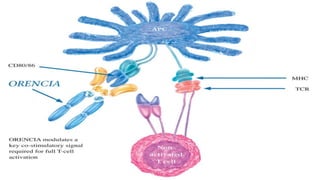
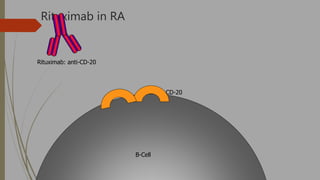
![Biologic therapies, or biologics
o Newer drugs that reduce RA inflammation in a more
highly targeted manner than the sDMARDs. These are
used when there is inadequate response with the
DMARDS
o Biologics are made through biotechnology and target very
specific proteins or cells that are involved in the
inflammatory process.
o Biologics have also been shown to help reduce the
progression of joint damage in RA.
o The currently available biologic therapies for RA must
either be injected under the skin [etanercept,
adalimumab, anakinra] or infused [infliximab, abatacept,
and rituxumab]).](https://image.slidesharecdn.com/dmards-170523190937/85/DMARDs-18-320.jpg)