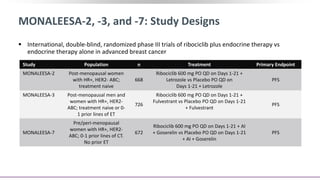
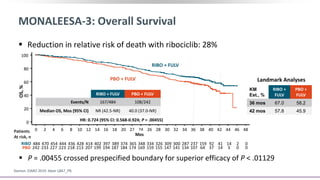
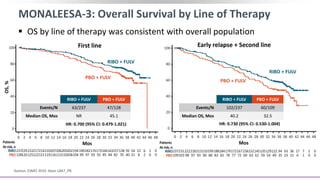
This document discusses a clinical trial evaluating the combination of ribociclib and endocrine therapy for pre/perimenopausal women with HR+, HER2- advanced breast cancer. The trial aimed to assess whether ribociclib plus an aromatase inhibitor and goserelin improved progression-free survival compared to placebo plus the same endocrine therapies. Key findings were that the combination led to a statistically significant improvement in progression-free survival. Overall survival data were also collected as a secondary outcome.

![Breast Cancer: Incidence
BC is the most common cancer in
women in the United States[1]
‒ Estimated 266,120 diagnoses in 2018,
representing 15.3% of all new cancers
HR+/HER2- BC is most common
molecular subtype[2]
~ 6% of patients present with
metastatic disease and up to 50%
with primary BC will later develop
metastatic disease[3]
‒ Currently, metastatic BC is incurable[3]
1. NIH SEER. Cancer stat facts: female breast cancer. 2. Howlader N, et al.
J Natl Cancer Inst. 2014;106. 3. Brufsky AM. Cancer Treat Rev. 2017;59:22-32.
Distribution of BC Molecular Subtypes
in United States, 2010[3]
HR+/HER2-
73%
TNBC
12%
HR+/
HER2+
10%
HR-/
HER2+
5%](https://image.slidesharecdn.com/hrher2neu-mbcppt-200905145742/85/Hr-her2-neu-mbc-ppt-2-320.jpg)

![G2
S
M
G1
pRB
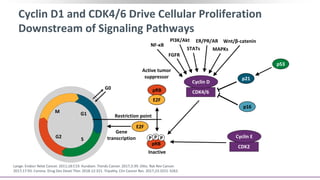
The Role of CDK4/6 in Breast Cancer
Growth of HR+ MBC is
dependent on cyclin D1, a direct
transcriptional target of ER
Cyclin D1 activates CDK4/6,
resulting in G1-S phase
transition and cell cycle entry[1]
Some cell-line models of
endocrine resistance show
dependence on cyclin D1 and
CDK4/6[2,3]
ERα Mitogenic
signaling
S phase transcription
program
G1/S transition
P P P
pRB
E2F
E2F
CDK1
Cyclin B
CDK1/2
Cyclin A
CDK2
Cyclin E
CDK4/6
Cyclin D](https://image.slidesharecdn.com/hrher2neu-mbcppt-200905145742/85/Hr-her2-neu-mbc-ppt-7-320.jpg)

![CDK4/6 Inhibitors: Comparison of Key Clinical
Characteristics
1. DeMichele A, et al. Clin Cancer Res. 2015;21:995-1001. 2. Hamilton E, et al. Cancer Treatment Rev.
2016;45:129-138. 3. Costa R, et al. Ann Oncol. 2017;28:44-56. 4. Infante JR, et al. Clin Cancer Res.
2016;22:5696-5705. 5. Barroso-Sousa R, et al. Breast Care. 2016;11:167-173. 6. Dickler MN, et al. ASCO
2016. Abstract 510.
Characteristic Palbociclib[1-3] Ribociclib[4,5] Abemaciclib[5,6]
Target (IC50, nM) CDK4 (11); CDK6 (15) CDK4 (10); CDK6 (39) CDK4 (2); CDK6 (10)
Route PO PO PO
Dose, mg 125 QD 600 QD Monotx: 200 BID
Combo w/ET: 150 BID
Schedule 3 wks on/1 wk off 3 wks on/1 wk off Continuous
Half-life, hr 27 32.6 17-38](https://image.slidesharecdn.com/hrher2neu-mbcppt-200905145742/85/Hr-her2-neu-mbc-ppt-10-320.jpg)
![PALOMA-1[1] PALOMA-2[2] MONALEESA-2[3,4] MONARCH-3[5] MONALEESA-3[6]
Study
design
Phase II
1st line
Phase III
1st line
Phase III
1st line
Phase III
1st line
Phase III
1st and 2nd line
Endocrine
partner
Letrozole Letrozole Letrozole
Letrozole
or anastrozole
Fulvestrant
CDK4/6
inhibitor
Palbociclib Palbociclib Ribociclib Abemaciclib Ribociclib
Patients, N 165 666 668 493 367
HR 0.49 0.58 0.56 0.54 0.57
PFS, mos 20.2 vs 10.2 24.8 vs 14.5 25.3 vs 16 NR vs 14.7 NR vs 18.3
ORR, % 56 vs 39 55.3 vs 44.4 52.7 vs 37.1 59 vs 44 40.9 vs 28.7*
Impact of CDK4/6 Inhibition on PFS: First-Line Setting
*ORR includes 1st and 2nd line patients.
1](https://image.slidesharecdn.com/hrher2neu-mbcppt-200905145742/85/Hr-her2-neu-mbc-ppt-11-320.jpg)


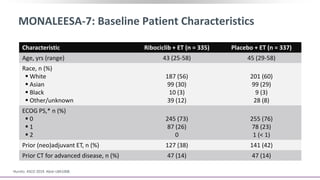
![MONALEESA-7: Background
Among women with breast cancer, younger patients generally have more aggressive disease and
poor prognoses vs older patients[1]
‒ Premenopausal women underrepresented in clinical trial populations
Ribociclib: CDK4/6 inhibitor FDA approved for HR+/HER2- advanced or metastatic breast cancer
with letrozole or another aromatase inhibitor as initial ET for pre/peri/postmenopausal women, or
with fulvestrant as initial ET or following PD on ET for postmenopausal women[2,3]
Unknown whether CDK4/6 inhibition + ET significantly prolongs OS in breast cancer; challenging to
assess in trials due to potential crossover between arms, variable treatment histories[4,5]
Phase III MONALEESA-7 trial showed significantly prolonged PFS with ribociclib + ET vs placebo + ET
as initial ET in pre/perimenopausal women with HR+/HER2- advanced breast cancer[6]
Current report presents protocol-specified interim analysis of OS in MONALEESA-7 trial[7,8]
1. Bardia. Clin Cancer Res. 2018;24:5206. 2. Ribociclib and letrozole PI. 3. Ribociclib PI. 4. Turner. NEJM. 2018;379:1926. 5. Hurvitz.
Cancer Treat Rev. 2011;37:495. 6. Tripathy. Lancet Oncol. 2018;19:904. 7. Hurvitz. ASCO 2019. Abstr LBA1008. 8. Im. NEJM. 2019;[Epub].](https://image.slidesharecdn.com/hrher2neu-mbcppt-200905145742/85/Hr-her2-neu-mbc-ppt-18-320.jpg)
![MONALEESA-7: Subsequent Anticancer Therapies and
Time to First Subsequent Chemotherapy
Im. NEJM. 2019;[Epub].
First Subsequent Therapy
Ribociclib + ET
(n = 335)
Placebo + ET
(n = 337)
Patients discontinuing
study tx, n
219 280
Received any
subsequent tx, n (%)
151 (68.9) 205 (73.2)
– CT alone, n (%) 49 (22.4) 80 (28.6)
– CT + hormone
therapy/other, n (%)
18 (8.2) 22 (7.9)
– Hormone therapy
alone, n (%)
49 (22.4) 57 (20.4)
– Hormone therapy +
other (no CT), n (%)
31 (14.2) 41 (14.6)
– Other, n (%) 4 (1.8) 5 (1.8)
– CDK4/6 inhibitor(s),
n (%)
22 (10.0) 52 (18.6)
Time to First Subsequent Chemotherapy
Patients at Risk, n
Events,
n
Median Time to
Subsequent CT, Mos
Ribociclib + ET
Placebo + ET
Patients,
n
335 95 NR
337 139 36.9
HR for death: 0.596 (95% CI: 0.459-0.774)
Ribociclib335 324307 299 288275 267 255247240231225216 206195 158125 90 54 35 21 5 2 0
Placebo 337 315 288 277261246232 223212204194181174 161147 119 86 67 42 20 11 6 1 0
Mos
Ribociclib + ET
Placebo + ET
OS
100
80
60
40
20
0
0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46](https://image.slidesharecdn.com/hrher2neu-mbcppt-200905145742/85/Hr-her2-neu-mbc-ppt-22-320.jpg)
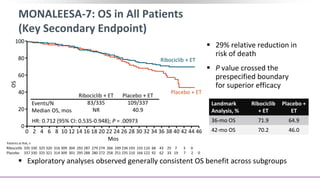
![MONALEESA-7: Conclusions
In this protocol-specified interim analysis of the phase III MONALEESA-7 trial for
pre/perimenopausal women with HR+/HER2- advanced breast cancer, the key secondary
endpoint of OS was significantly prolonged with ribociclib + ET vs placebo + ET alone
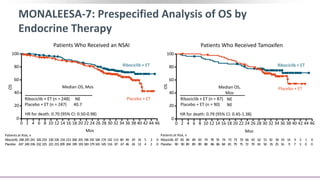
‒ Median OS: NE vs 40.9 mos (HR: 0.71; 95% CI: 0.54-0.95; P = .00973)
‒ Median OS with an NSAI: NE vs 40.7 mos (HR: 0.70; 95% CI: 0.50-0.98)
Patients still receiving study treatment: ribociclib arm, 35%; placebo arm, 17%
Ribociclib + ET was associated with benefit that continued beyond initial treatment, as
shown in prolonged time to subsequent CT and PFS during subsequent therapy or death
from any cause
According to study investigators, this is the first report of significantly prolonged OS with a
CDK4/6 inhibitor + ET in patients with HR+/HER2 advanced breast cancer
‒ MONALEESA-7 the only trial to date to assess CDK4/6 inhibitors in only premenopausal women
Hurvitz. ASCO 2019. Abstr LBA1008. Im. NEJM. 2019;[Epub].](https://image.slidesharecdn.com/hrher2neu-mbcppt-200905145742/85/Hr-her2-neu-mbc-ppt-23-320.jpg)