Embed presentation
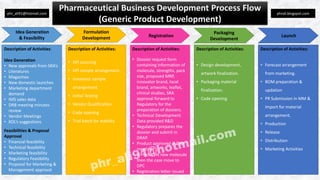

The document outlines the pharmaceutical business development process, detailing activities related to idea generation, regulatory approvals, financial and technical feasibility, and marketing demands. It discusses the stages of API sourcing, testing, dossier preparation, and product registration, culminating in product launch and distribution. Overall, it emphasizes a systematic approach to developing generic pharmaceutical products.
