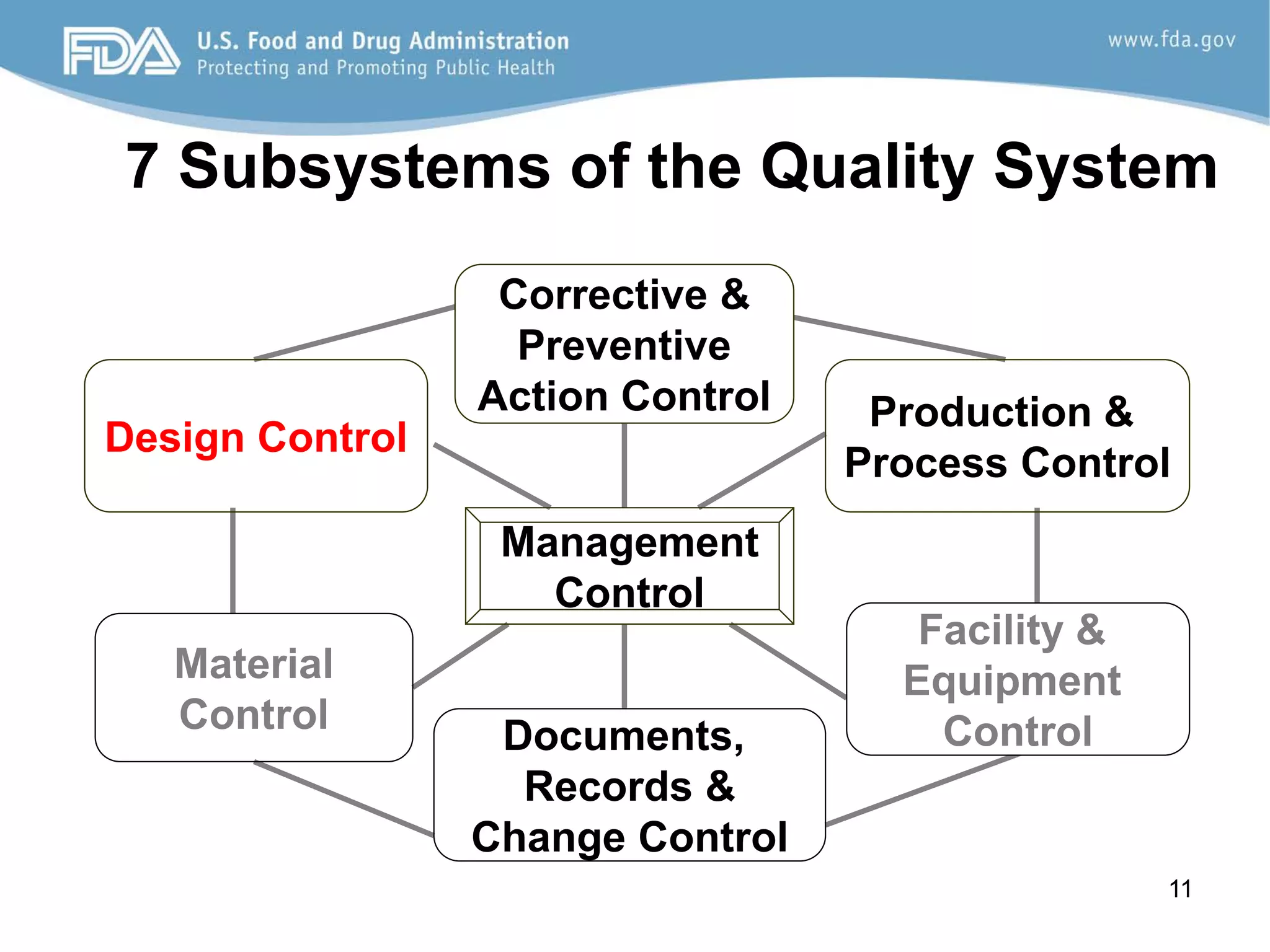
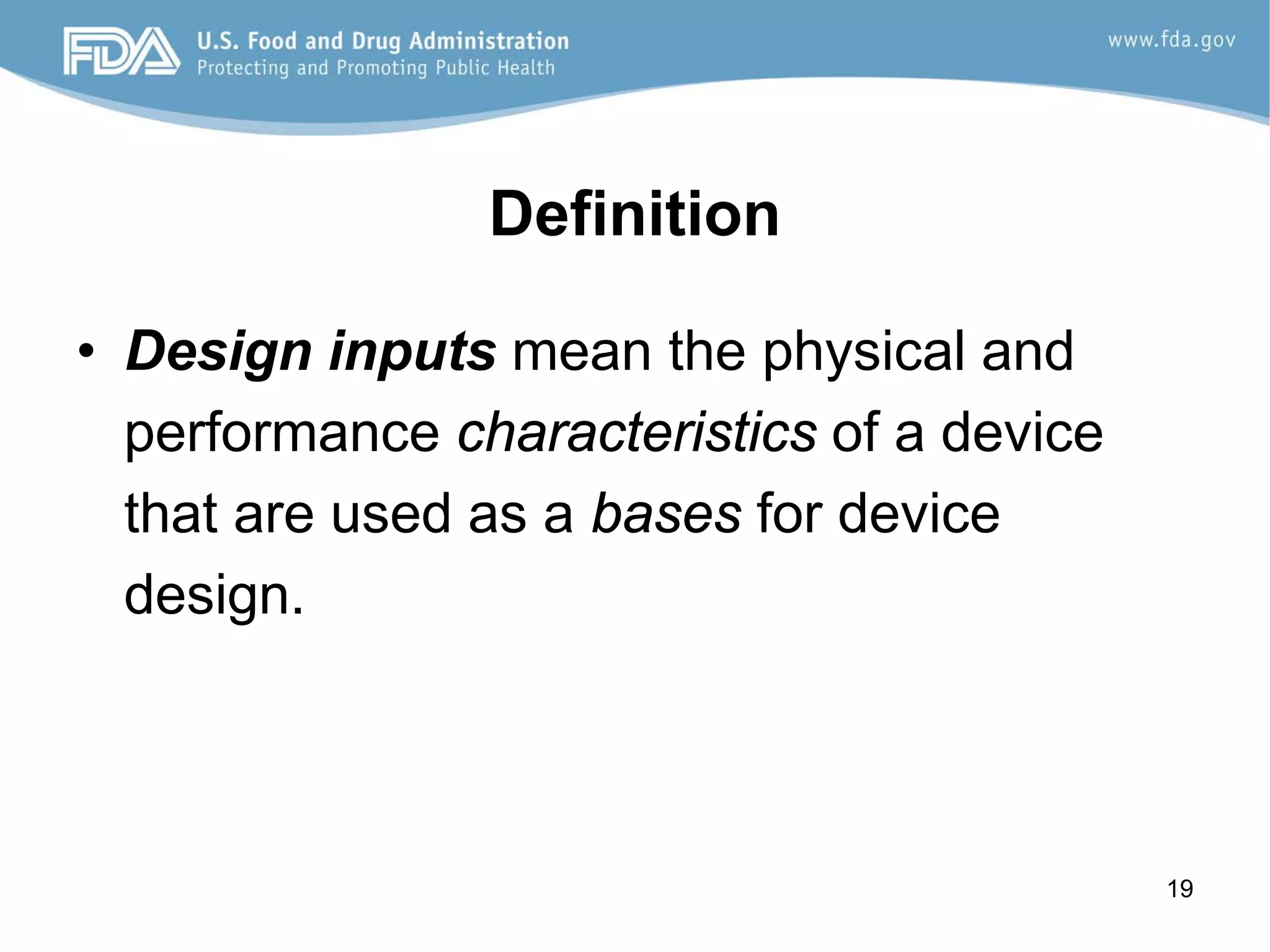
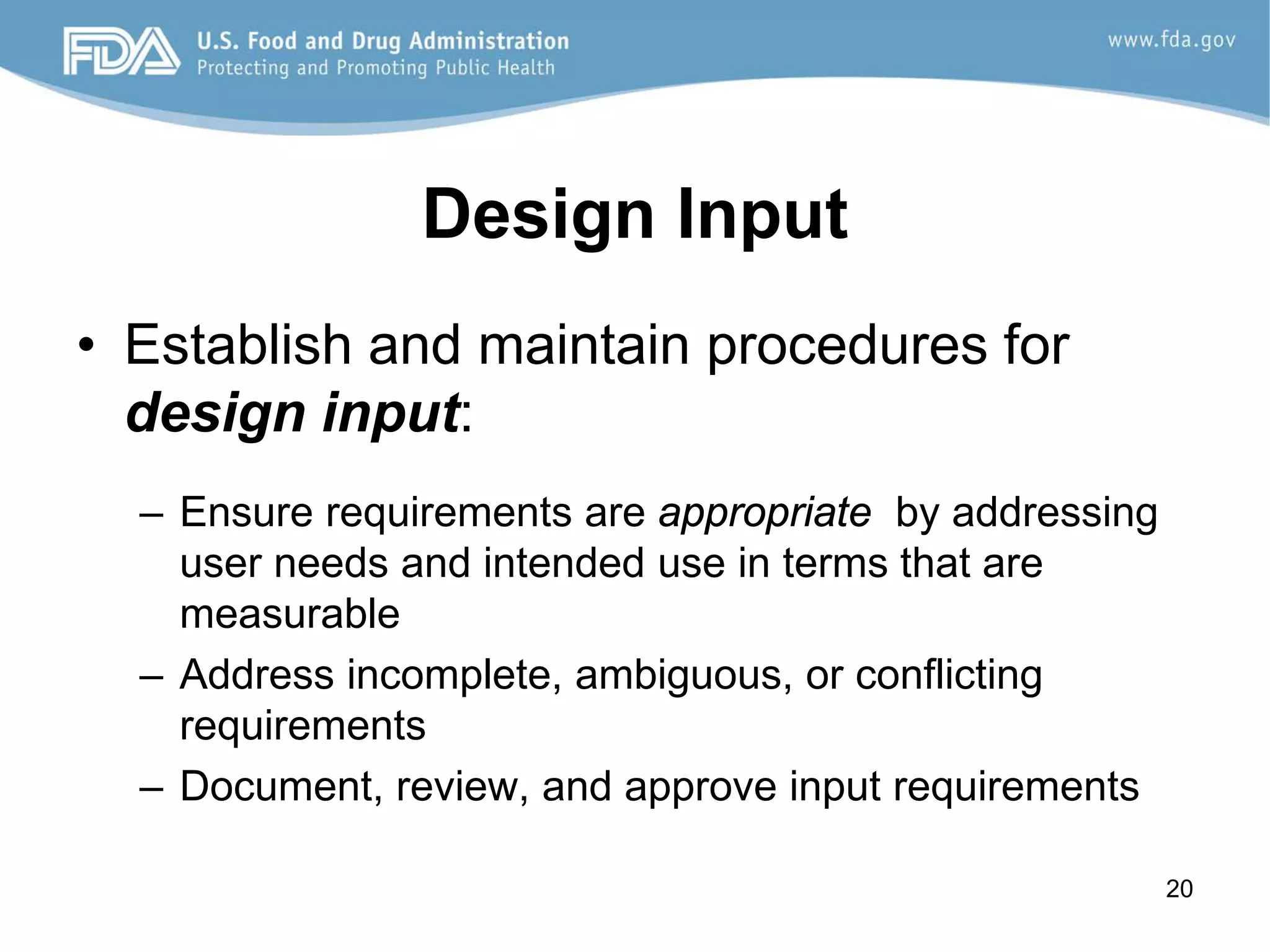
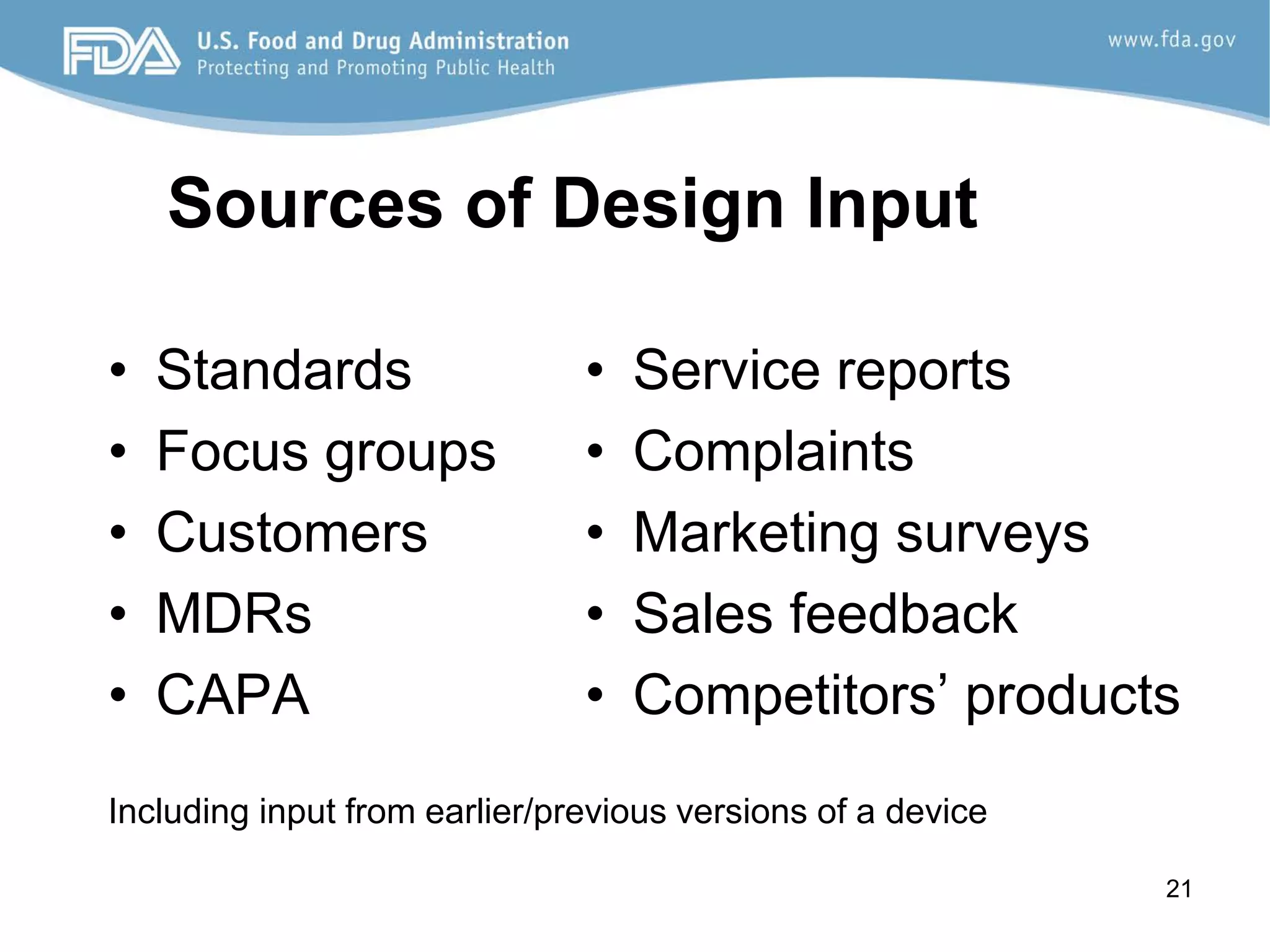
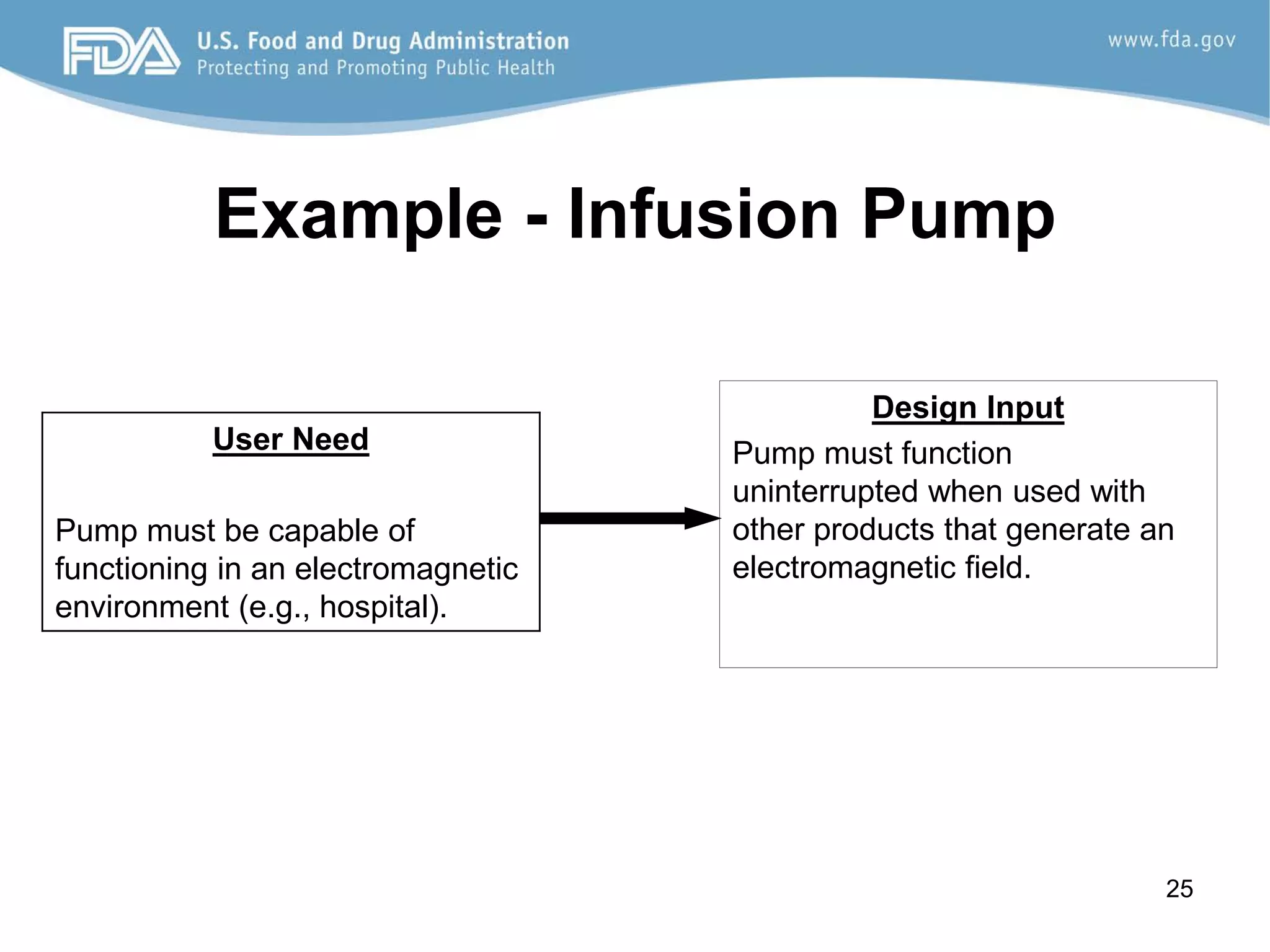
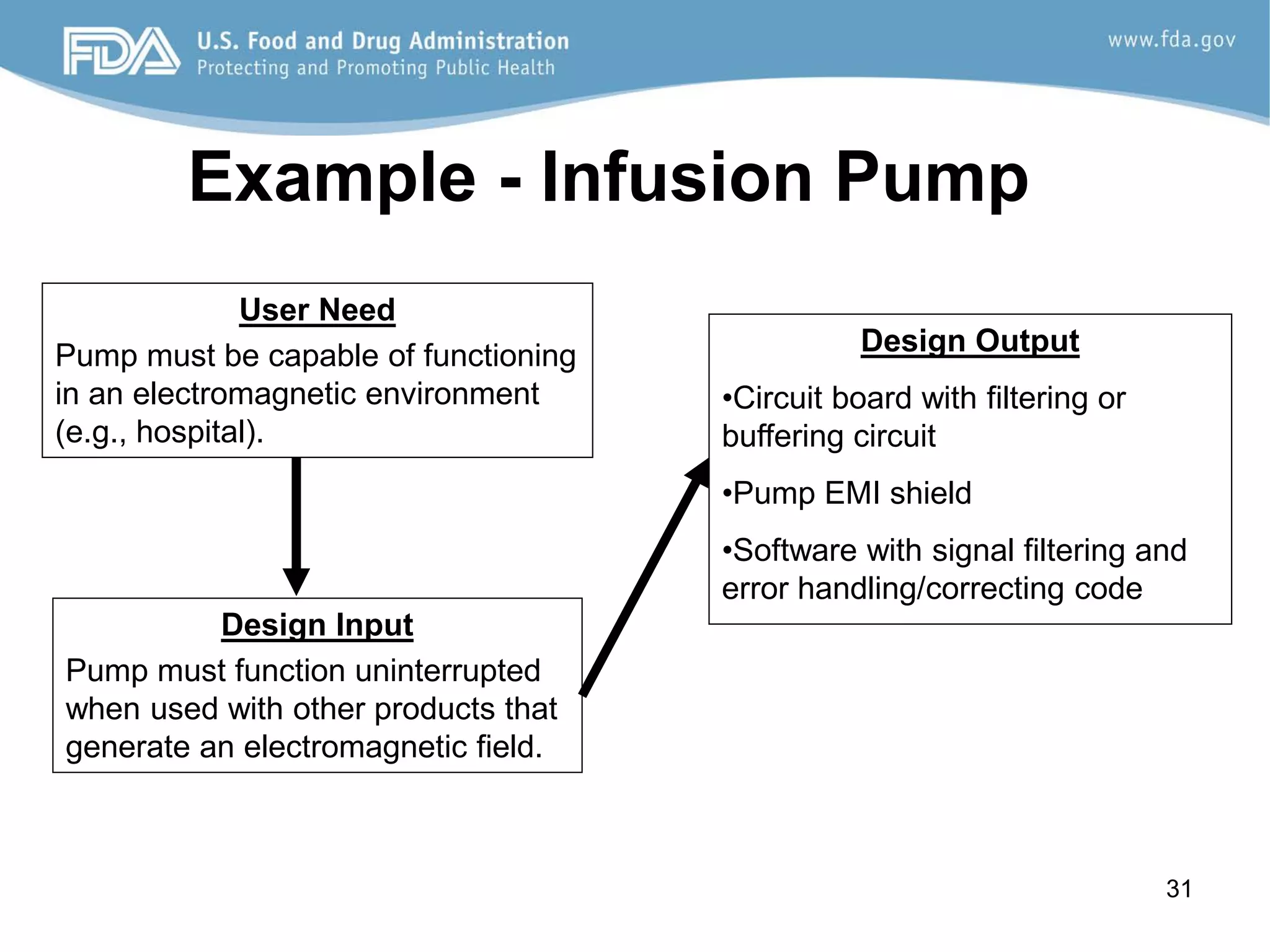
The document discusses design controls, which are a set of quality practices and procedures incorporated into the design and development process to control the design process and ensure medical device specifications meet user needs and intended use. It provides an overview of the seven key elements of design controls according to FDA regulations: design and development planning, design input, design output, design review, design verification, design validation, and design changes. It emphasizes that design controls are important for medical device safety and quality.