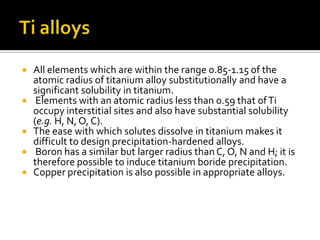
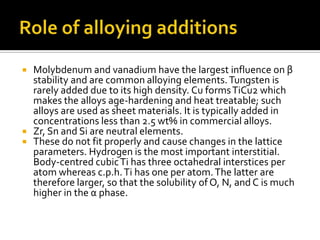
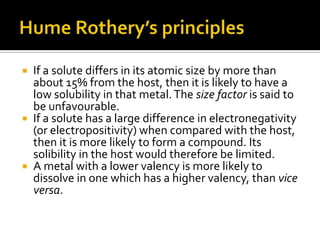
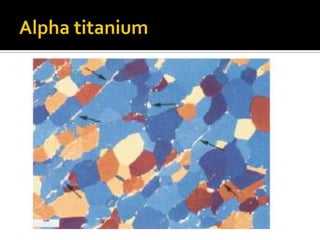
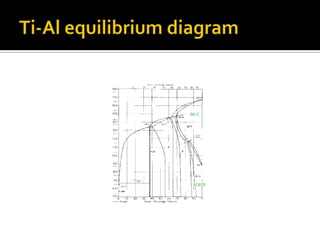
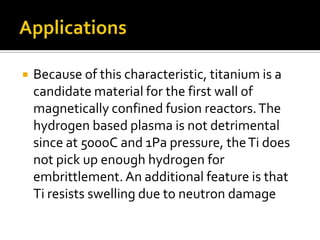
Titanium and its alloys have a high strength-to-weight ratio. Titanium is light, strong, ductile when pure, and has a high melting point. It is the seventh most abundant metal. Commercially pure titanium has a density about 45% lighter than steel. Titanium is resistant to corrosion and has good performance in seawater environments. Around 50% of titanium produced is used as the alloy Ti-6Al-4V. Titanium exists in both a hexagonal alpha phase and body-centered cubic beta phase, and alloys can contain mixtures of these phases. Common applications of titanium alloys include jet engines, implants, and marine applications due to its corrosion resistance and strength.



![TitaniumAtomic number = 22Atomic weight = 47.9Electronic configuration + [Ar]4S2 d 2Atomic radius = 144.2Melting point = 1668Boiling point = 3287Oxidation state = 4,3,2](https://image.slidesharecdn.com/titaniumanditsalloys-pptshow-110514110959-phpapp02/85/Titanium-and-its-alloys-ppt-show-4-320.jpg)





![Historical backgroundPure metallic titanium (99.9%) was first prepared in 1910 by Matthew A. Hunter by heating TiCl4 with sodium in a steel bomb at 700 – 800 °C in the Hunter process.[2] Titanium metal was not used outside the laboratory until 1946 when William Justin Kroll proved that it could be commercially produced by reducing titanium tetrachloride with magnesium in what came to be known as the Kroll process](https://image.slidesharecdn.com/titaniumanditsalloys-pptshow-110514110959-phpapp02/85/Titanium-and-its-alloys-ppt-show-15-320.jpg)