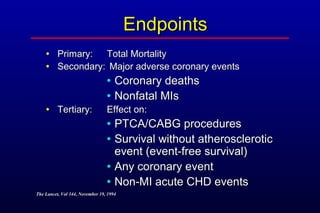
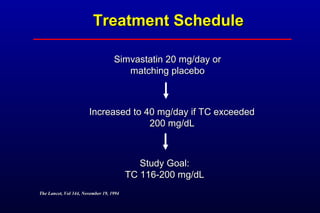
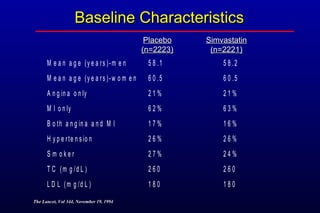
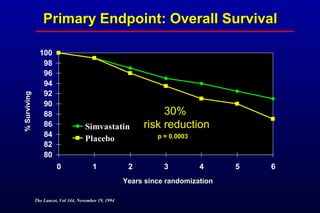
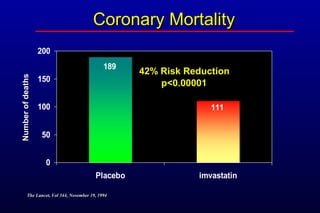
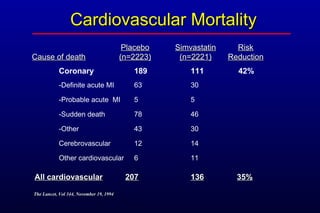
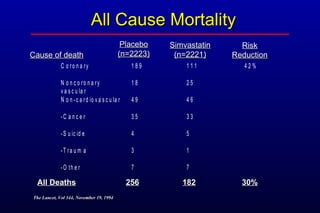
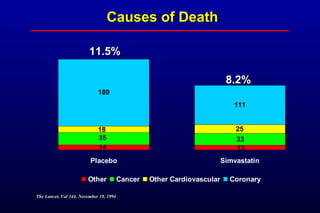
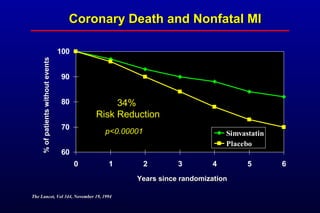
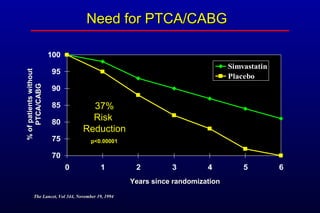
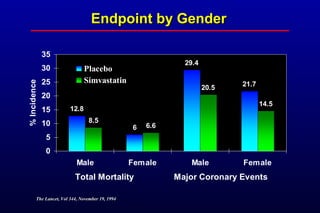
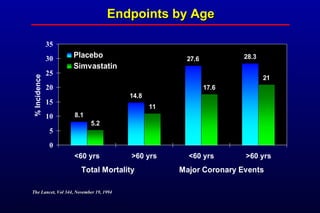
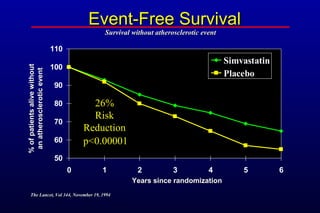
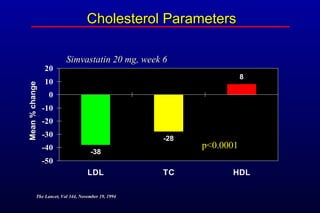
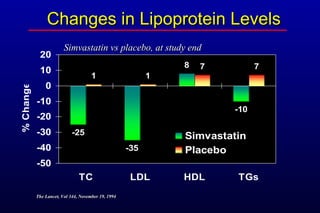
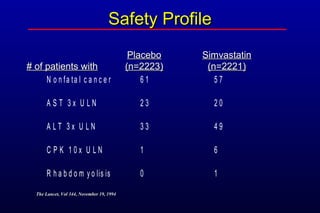
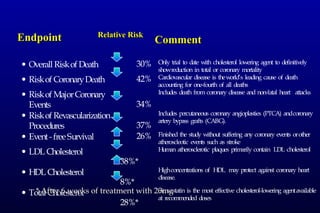
This randomized controlled trial investigated the effects of long-term simvastatin therapy in reducing mortality and coronary events in 4,444 patients with coronary artery disease. Patients received either simvastatin 20-40 mg/day or placebo. The study found that simvastatin reduced total mortality by 30%, coronary mortality by 42%, and major coronary events such as heart attack by 34% compared to placebo. Simvastatin also reduced the need for procedures like angioplasty or bypass surgery by 37% and improved long-term survival free from cardiovascular events by 26%. Simvastatin significantly lowered LDL cholesterol and total cholesterol levels.