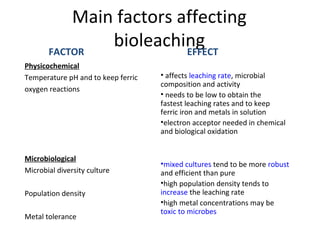
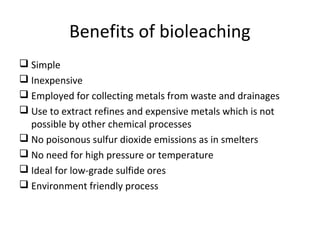
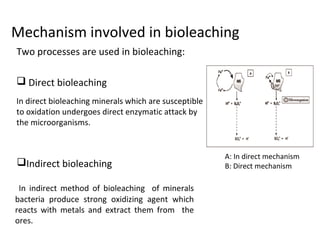
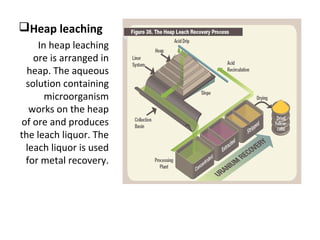
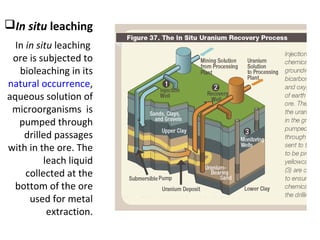
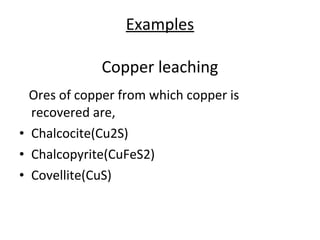
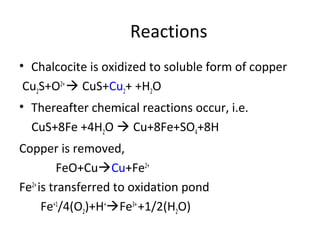
Bioleaching uses microorganisms like bacteria and fungi to extract metals from ores and concentrates. It is a simple and environmentally friendly process that has been used for over 3000 years to extract copper. Common microbes used are mesophilic and moderately thermophilic bacteria. Bioleaching involves both direct and indirect mechanisms. Direct bioleaching involves enzymatic attack of bacteria on susceptible minerals while indirect uses bacteria to produce strong oxidizing agents. Commercial bioleaching uses heap and in-situ leaching with controls on pH, temperature, oxygen and carbon dioxide to optimize the slow natural process. It is used to extract copper, gold, silver, uranium and other metals.




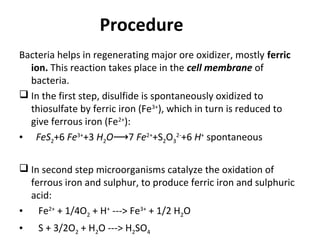





![• Uranium leaching is an indirect process
• When T. ferrooxidans are involved in uranium extraction, they
do not directly attack on ore but on the iron oxidants.
• The pyrite reaction is used for the initial production of Fe
Reaction;
2FeS+H2O+7 ½[O2] Fe2[SO4]3+ H2SO4](https://image.slidesharecdn.com/bioleachingitstechniqueandapplications-170508131521/85/Bioleaching-its-technique-and-applications-18-320.jpg)