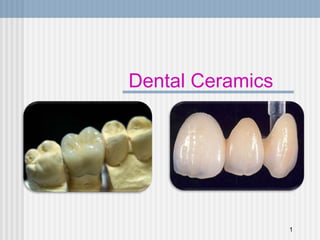
318500924-6-DENTAL-CERAMICS-PPT.ppt
- 2. Ceramic is derived from the Greek word “keramikos” -‘earthen’. Ceramic is derived from Sanskrit word meaning burnt earth. Introduction 2 Although many advances have been made in composites and glass ionomers, ceramic material holds a special place in dentistry. Its color, translucency and vitality cannot be matched by any material.
- 3. Compounds of one or more metals with a non metallic element that may be used as a single structural component or as one of the several layers that are used in the fabrication of a ceramic based prosthesis . (GPT- 7) Ceramics 3 are composed of essentially the same materials, the principle difference being in the proportion of the primary ingredients (such as feldspar, silica and kaolin/ clay) and firing procedures (temperature, method etc). All porcelains are ceramics, but not all ceramics are porcelains. Porcelain
- 4. 4
- 5. History of porcelain used as a dental material goes back nearly 250 years. 1728 - the use of porcelain in dentistry was first mentioned by Pirre Fauchard. 1774 – Alexis Duchateau, with the assistance of a Parisian dentist Nicholas Dubois de Chemant, made the first successful porcelain dentures replacing the stained and malodorous ivory prostheses of Duchateau History…….. 5
- 6. 1806 to1808 - Fonzi an Italian dentist introduced the first porcelain teeth that contained embedded platinum pins. But they never met with great approval because of their brittleness and opacity. He also used metal oxides to produce 26 shades of color in porcelain. 1825- Samuel Stockton began fabrication of fused porcelain teeth in Philadelphia. His initials were represented in the name of the S.S.White company. 1837 – John Murphy of London introduced the plantium foil technique History…….. 6
- 7. 1884 – Dr Charles H.Land pioneered the development of the first glass furnace for fusing porcelain. 1887 – Dr C.H.Land of Detroit developed the first porcelain jacket crown (PJC) using the Platinum Foil Matrix technique. 1894 – Levitt Ellsworth Custer developed the first electric furnace for porcelain. 1903 - Dr.Charles Land introduced first ceramic crowns to dentistry. History…….. 7
- 8. 1910 – High fusing electric furnaces (fusion at 20000 F) were recommended to minimize the firing shrinkage and application of hydrofluoric acid to the fitting surface to produce a ‘honeycomb’ appearance to enhance retention. 1923 - Wain - inlays and onlays using dental porcelain. 1940 - with advent of acrylics PJC lost popularity. 1957 – S. D. Stookey invented glass-ceramic. History…….. 8
- 9. History…….. 1958 - Vines et al, introduced finer porcelain powders for vacuum firing. It was the first major improvement in the esthetics, because it increased the translucency of all-porcelain crowns. 9 1962- Weinstein et al patented formulations of feldspathic porcelain and alloys that bonded chemically & were thermally compatible with feldspathic porcelains. 1963 - first commercial porcelain developed by Vita Zahnfabrik.
- 10. History…….. 10 1965- McLean & Hughes introduced dental aluminous core ceramic with significant improvement in fracture resistance. 1968 – W.T. MacCulloch fabricated denture teeth from a glass- ceramic. He suggested the possibility of using glass-ceramics in inlays and crowns. 1976 – McLean & Sced developed the platinum bonded alumina crown. The attachment of aluminous porcelain to the platinum was achieved by surface coating of the metal with a thin layer of tin.
- 11. 1983 – Sozio & Riley first described shrink-free ceramics (marketed as Cerestore), which was followed by development of injection-molded castable glass-ceramic by the University of Zurich (marketed as IPS Empress). 1984- Adair & Grossman introduced Dicor glass-ceramic. 1985 – First CAD/CAM crown was publically milled and installed in the mouth 1986 – The first generation CEREC 1 (Siemens) CAD/CAM system was introduced. History…….. 11
- 12. 1988 – Michael Sadoun first introduced In-ceram, a glass-infiltrated aluminous porcelain. 1989 – The concept of all-ceramic post & core was introduced using Dicor glass-ceramic initially, followed by In-cream, IPS Empress and Zirconica ceramics. History…….. 12 1993 – The Procera CAD/CAM system was developed by Andersson M. & Oden by a co- operative effort between Nobel Biocare and Sandvik.
- 13. 1994 – The second generation CEREC 2 (Siemens/Sirona) CAD/CAM system was presented. Late 1990’s – IPS Empress 2, a second generation pressable ceramic made from lithium-disilicate frame work with an apatite layered ceramic was introduced 1999 – IPS SIGN (Ivoclar AG), a feldspar-free fluorapatite glass ceramic system for use in metal-ceramics was presented. History…….. 13
- 14. History…….. 2000 – two dimensional CEREC 3 was presented. 2002 - Lava uses a laser optical system to digitize information from multiple abutment margins 14 2005 – The three dimensional CEREC 3D was presented. Scanning and designing 3 dimensional viewing Milling
- 15. According to Type 1. Feldspathic porcelain 2. Leucite-reinforced porcelain 3. Aluminous porcelain 4. Alumina 5. Glass-infiltrated alumina 6. Glass-infiltrated spinel 7. Glass-ceramic. According to Processing Method 1. Sintering 2. Casting 3. Machining K.J. Anusavice, 1996, Phillips 10th edition) Classification of Dental Ceramics 15
- 16. According to Use or Indications Denture teeth, inlay, onlay, Ceramic brackets for orthodontic treatment. Classification…… Anterior Crowns Posterior Crowns Veneers Fixed Partial Dentures Post & cores 16
- 17. According to Substructure Material 1. Cast metal 2. Swaged metal 3. Glass-ceramic 4. CAD-CAM porcelain 5. Sintered ceramic core. (By K.J. Anusavice, 1996, Phillips 10th edition). 17
- 18. 1. Air fired. 2. Vacuum fired According to Method of Firing 1. Ultra low fusing (<850oC) 2. Low fusing (850oC -1100oC) 3. Medium fusing (1100oC -1300oC) 4. High fusing (>1300oC) According to Firing Temperature 1. Non-Crystalline Ceramics e.g.: Feldspathic porcelain 2. Crystalline Ceramics e.g.: Aluminous porcelain, Glass-Ceramics According to Microstructure 18
- 19. 1. Core porcelain 2. Opaque porcelain 3. Body (dentin) porcelain 4. Gingival, cervical or neck porcelain 5. Enamel (incisal) porcelain 6. Color frits (pigments) 7. Glazed porcelains According to Varieties Used/ Application Classification…… 19
- 20. Classification…… According to method of fabrication -(Marc Rosenblum & Alan Schulman JADA March 1997). Cast metal systems : eg: Vita Metall Keramik (VMK 95) Non- Cast Metal Systems Foil Crown Systems /All – Ceramic Systems 1. Conventional Powder – Slurry Ceramics 2. Castable Ceramics 3. Machinable Ceramics 4. Pressable Ceramics 5. Infiltrated Ceramics 20
- 21. 1.Conventional Powder – Slurry Ceramics condensing & sintering. 1. Alumina reinforced Porcelain e.g. Hi-Ceram 2. Magnesia reinforced Porcelain e.g. Magnesia cores 3. Leucite reinforced (High strength porcelain) e.g. Optec HSP 4. Zirconia whisker – fiber reinforced e.g. Mirage II 5. Low fusing ceramics 21
- 22. 2. Castable Ceramics casting & ceramming 1. Flouromicas e.g. Dicor 2. Apatite based Glass-Ceramics e.g. Cera Pearl 3. Other Glass-Ceramics e.g. Lithia based, Calcium phosphate based 22
- 23. 3. Machinable Ceramics Milling & machining Analogous Systems Grinding techniques : a) Mechanical e.g. : Celay b) Automatic e.g. Ceramatic II. DCP Erosive techniques: a) Sono-erosion e.g. Erosonic b) Spark-erosion e.g. Procera Digital systems (CAD / CAM): Direct e.g. Cerec 1 & Cerec 2 Indirect e.g. Cicero, Denti CAD 23
- 24. 4. Pressable Ceramics pressure molding & sintering 1. Shrink-Free Alumina Reinforced Ceramic (Injection Molded) E.g. Cerestore / Alceram 2. Leucite Reinforced Ceramic (Heat – Transfer Molded) E.g. IPS Empress, IPS Empress 2, Optec OPC. 24
- 25. 5. Infiltrated Ceramics slip-casting, sintering & glass infiltration 1) Alumina based e.g. In-Ceram Alumina 2) Spinel based e.g. In-Ceram Spinel 3) Zirconia based e.g. In-Ceram Zirconia 25
- 26. Ingredients Wt % Function Feldspar 60-80 Basic glass former Alumina 8-20 Strengthener, glass former, opacifier Kaolin 3-5 Binder during firing Quartz (crystalline silica) 15-20 Filler Boric oxide 2-7 Glass modifiers, flux Oxides of Na, K, Ca 9-15 Glass modifiers, interrupter, fluxes Metallic pigments <1% Color matching Oxides of Zr, Sn, Ba, Ti, B Trace As color pigments & shade Composition of Dental Ceramics 26
- 27. Feldspar These are a group of naturally occurring minerals, which are complex alkali aluminium silicate. Types of feldspar: Soda feldspar – Sodium alumina ( Na2O Al2O3, 2SiO2, 2H2O) decreases fusion temperature Potash feldspar – Potassium aluminium silicate ( K2O, Al2O3, 6SiO2) increases the viscosity of glass. Composition ….. 27 Proper potash content decreases the danger of excessive pyroplastic flow during firing of porcelain, which could otherwise result in rounding of the edges, loss of form ,shape; and the obliteration of surface characteristics which contribute to a life like appearance.
- 28. Role of feldspar Glass phase formation: During firing, the feldspar fuses and forms a glassy phase that softens and flows slightly allowing the porcelain powder particles to coalesce together, thus acts as a matrix and binds silica and kaolin. Composition ….. 28 It is a basic glass former Leucite formation Between 1150o & 1530oc it undergoes incongruent melting and forms crystals of leucite. Leucite is a potassium aluminum silicate with large coefficient of thermal expansion
- 29. Function of Leucite To raise the coefficient of thermal expansion of porcelain and bring it closer to that of the metal substrate; consequently increasing the hardness and fusion temperature. Strengthening of porcelain e.g. Optec HSP, Cerinate, & IPS Empress. 29 Composition …..
- 30. Increases the mouldability of the plastic porcelain Acts as a binder and helps in maintaining the shape of the unfired porcelain during firing. At high temperature, it fuses and reacts with other ingredients to form the glassy matrix. Composition ….. Kaolin 30 Hydrated aluminum silicate. Opacity even when present in very small quantities. Drawback
- 31. It is basically a glass consisting of 3-dimensional network of silica with a very high fusion temperature. Composition ….. Quartz (Crystalline Silica) 31 Acts as a filler Provides strength and hardness to porcelain. Because it has a high melting point, it maintains the form (shape) of a freestanding object during firing. Functions
- 32. Glass Modifiers or Fluxes Can be defined as elements that interfere with the integrity of the SiO2 (glass) network and alter their three-dimensional state. Purpose To lower the fusion temperature of a glass by reducing the amount of cross-linking between the oxygen and glass forming elements. Increase the flow of porcelain during firing. Composition ….. 32 e.g. Alkali metal ions such as Na, K or Ca (usually as carbonates). If concentration is too high: It reduces chemical durability of the glass. (resistance to attack by water, acids and alkalis) It causes the glass to crystallize or devitrify.
- 33. Alumina (Aluminum oxide) One of the hardest and strongest oxides of aluminium Increases viscosity of porcelain during firing. Gives strength and opacity to the material. 33 Boric Oxide Boric Oxide (B2 O3) although a powerful flux (glass modifier), it can also act as a glass former and form its own glass network, producing Boron Glasses. 12%- above which the less stable form BO3 takes over.
- 34. Water Water is an important glass modifier. The hydronium ion, H3O can replace Na or other metal ions in a ceramic that contains glass modifiers. Significance This replacement is responsible for the phenomenon of “slow crack growth” of ceramics that are exposed to tensile stresses and stored in moist environment Composition ….. 34
- 35. Coloring Agents Dental porcelains colored by the addition of concentrated color frits (generally metallic oxides) into the basic glass. The glass thus obtained will be highly color saturated and when ground to a fine powder, can be used in small amounts to modify the uncolored porcelain powder. Composition ….. 35 Pink- Chromium or chrome-aluminia Yellow – indium Blue- Cobalt salts in the form of oxide are useful in developing of the enamel shades Grey- Iron oxide (black) or platinum Grey: used for producing enamels or grayer section of the dentin colors, and also for an effect of translucency.
- 36. Green- Chromium oxide: is generally avoided since green is the characteristic color of glass. Other pigments used may be – Titanium oxide –yellow brown, manganese oxide- lavender, iron/nickel oxide- brown, copper oxide – green. Composition ….. 36
- 37. Opacifying Agents Reduces the translucency of porcelain Produces dentin colors in particular, which requires greater opacity than that of enamel colors. The common metallic oxides used are – Cerium oxide, Titanium oxide,Tin oxide, and Zirconium oxide (ZrO2)- most popularly used opacifying agent. 37 consists of a metal oxide.
- 38. Mode of Supply Porcelain kit consists of: Fine ceramic powders of different shades of enamel, dentin, core/opaque Special liquid/ distilled water- vehicle/medium for ceramic powder (binder) Stains or color modifiers Glazes 38 Various enamel, dentin and opaquer porcelains
- 39. Methods of fabricating ceramic restorations Condensing and Sintering, Casting & Ceramming Milling (Machining) by mechanical and digital systems. Pressure molding & Sintering Slip casting, Sintering & Glass infiltration 39
- 40. Condensation Sintering Glazing Cooling. Fabrication of Porcelain 40 However, fabrication of a conventional porcelain restoration is basically composed of the following stages:
- 41. Mixing of powder and liquid 41
- 42. Condensation Process of packing the powder particles together & removing excess water is known as condensation. Porcelain powder particles within the mass are closely packed in order to reduce volume shrinkage of porcelain & minimize porosity in the fired porcelain. Porcelain should not dry out as the porcelain is held together due to surface tension. 42
- 43. 1. Spatulation 2. Brush technique 3. Vibration 4. Ultrasonic Condensation………… Methods of condensation 43 Condensation of porcelain slurry on a metal framework
- 44. Article is carefully smoothened with a spatula when extra water from inside comes to the surface by capillary action which is removed by blotting paper or linen cloth. 44 Condensation………… Spatulation This method employs the addition of dry porcelain powder on the surface by a brush to the side opposite of wet porcelain to absorb the moisture. As the water is drawn towards the dry powder, the wet particles are pulled together. Brush technique
- 45. This method used mild vibration to pack the wet powder densely on the underlying framework. The excess water is blotted away with a clean tissue An electrically operated brush called the Vibra Brush can also be used in this technique, which although very efficient, lacks the flexibility offered by the light weight hand held sable brush. 45 Condensation………… Vibration Method Ultrasonic method Ultrasonic vibrations are transmitted electrically & water coming out is removed using blotting paper or linen cloth.
- 46. Sintering The process of fusing the condensed mass is known as firing/ sintering. Porcelain firing unit (muffle chamber) is preheated to 650oC & article to be fired is placed in a fire clay tray & then placed on a platform. Preheating the porcelain by placing it in front of the muffle is essential. Direct heating causes rapid steam production that breaks up the mass. 46
- 47. Platform is raised & article is held inside muffle chamber for 5 min. (remaining water is converted into steam & comes out). Door of muffle chamber is closed & evacuated by connecting it to a vacuum pump. Temperature is gradually raised to firing temperature of porcelain. Muffle Chamber 47 Firing procedure………
- 48. Shrinks 30-40% by volume. During firing there is partial fusion of particles at their point of contact. As the temperature is raised the fused glass gradually flows to fill up air spaces. Vacuum firing is done to reduce porosity in porcelain. 48
- 49. Stages of Maturity Stages are known as bisque/biscuit stages. Different stages are: Low bisque stage Medium bisque stage High bisque stage. Less the number of firings, higher is the strength and better is the esthetics. Firing procedure……… 49
- 50. As temperature rises, surface of the particles begin to soften & these loose particles just begin to join. No volume shrinkage. If firing is stopped at this stage, particles form a porous mass. Weak & friable Opaque Used in glass infiltrated ceramics- Inceram 50 Firing procedure……… Low Bisque Stage
- 51. On further heating, more softening of particles takes place & they begin to melt. Better cohesion. Slight volume shrinkage. Reduced although still porous Moderate strength Less opaque and color has developed 51 Firing procedure……… Medium Bisque Stage
- 52. Further heating causes melting of all particles producing complete cohesion & maximum volume shrinkage. Porosity absent High Strength Relatively smooth surface with a light sheen Color & translucency developed 52 Firing procedure……… High Bisque Stage As liquid is highly viscous, it retains its shape for some time If heating is prolonged, liquid gradually flows under gravity i.e. pyroplastic flow, & article looses sharp corners & its shape. Firing is discontinued usually at this stage for complete melting.
- 53. Glazes Colorless porcelain applied to the surface to give it a glossy lifelike appearance. Does not contain opacifiers. Low fusion temperature. Contain lot of glass modifiers. 53 Glazing To remove surface cracks & improve the flexure strength Two types : Auto glazing or Self glazing Over glazing
- 54. Auto glazing or Self glazing Temperature of porcelain is quickly raised to melt surface particles which flow & fill all the microcracks. Over glazing A thin layer of transparent glaze porcelain of lower fusion temperature is coated on body porcelain & is then fired at lower temperature only to melt outer layer of glaze porcelain which flows into surface cracks. 54 Glazing……
- 55. Cooling of the fired article Muffle chamber is gradually cooled according to manufacturer instructions for porcelain to undergo uniform shrinkage to minimize formation of micro cracks Platform is then brought down & the article is removed. 55 Surface microcracks can be filled by the application of Glaze
- 56. Excellent esthetic properties :: suitable stains can be applied :: color parameters (hue, chroma & value) are permanent. Color stability Chemically inert, excellent biocompatibility Chemical stability: insoluble and impermeable to oral fluids, chemical degradation by fluoride attack. 56 Properties
- 57. Low coefficient of thermal expansion, nearly same as tooth enamel 6.4 -7.8 x 10-6/oc. Dimensional stability: stable after firing Low thermal conductivity Compressive strength: good 480 MPA. Greater surface hardness (460 KHN) than tooth enamel (343 KHN) : abrasion & wearing of opposing natural tooth and metal restorations 57 Properties
- 58. Brittle Tensile strength - 35-50 MPA Modulus of elasticity: low 40 GPa Shear strength: low 110 MPa. Shrinkage Volumetric - 35 – 45 %, Linear - 11 – 14 % Minimized by using lesser binder, proper condensation, build – up of restoration 1/3rd larger than original size and firing in successive stages. 58 Properties
- 59. Griffiths Flaw Crack Growth Sintering Process Why are Ceramics weak ? On moisture exposure crack growth is accelerated 1. Brittle – Covalent bonds 2. Inherent flaws 3. > # in moist environment 59 D.W. Jones- 1983
- 60. In ceramics, micro cracks are caused by The condensation, melting and sintering process. The high contact angle of ceramics on metal. Differences in the coefficient of thermal expansion between alloy or core and veneers. Grinding and abrasion. Tensile stresses during function. 60
- 62. 62 1. Methods of Strengthening brittle materials 2. Methods of designing components to minimize stress concentrations and tensile stresses According to K. J. Anusavice (Phillip’s Science of D Materials, 1996)
- 63. A. Methods of strengthening brittle materials 1.Ion exchange 2.Thermal tempering 3.Thermal compatibility B) Methods of designing components to minimize stress concentrations and tensile stresses 1. Reducing stress raisers 2. Minimize tensile stresses Residual compressive stresses Interruption of crack propagation Transformation toughening Particle stabilized zirconia Dispersion of crystalline phase Al, dicor 63
- 64. This is one of the widely used methods of strengthening glasses and ceramics. Strengthening is gained by the fact that, residual stresses must first be negated by developing tensile stresses before any net tensile stress develops. 64 Development of Residual Compressive Stresses A. Methods of strengthening brittle materials
- 65. Smaller Na+ ions are exchanged by K+ ion which are 35% bigger. This replacement by ion exchange introduces large residual compressive stresses (roughly 700mpa/1,00,000 psi). Squeezing of K into a smaller space, termed as stuffing. Soda feldspathic porcelain is kept immersed in molten KNO3 solution for 20-30 min. 65 Development of residual compressive stresses……….. Ion exchange or chemical tempering Alumina reinforced materials, Dicor glass-ceramic core and some conventional feldspathic porcelains with high potash feldspar content. Limitation
- 66. It creates residual compressive stresses by rapidly cooling (quenching) the surface of the object while it is still hot, and in the molten state. This rapid cooling produces a skin of rigid glass surrounding a molten core. As the molten core solidifies, it shrinks, creating residual compressive stresses within the outer surface. 66 Development of residual compressive stresses……….. Thermal tempering
- 67. This method is used to strengthen glasses used for automobile windows and windshield, sliding glass doors and diving masks. For dental applications, it is more effective to quench hot glass- phase ceramics in silicone oil or other special liquids rather than using air jets that may not uniformly cool 67 Development of residual compressive stresses……….. Thermal tempering
- 68. Veneering ceramic with two or more layers of ceramic compositions having slightly different coefficient of thermal expansions; one layer contracting slightly more than other layers thus inducing residual compressive stresses. eg. Inceram, Metal-ceramic restorations On cooling metal contracts more than ceramic, thus leaving the outer layer of ceramic in residual compressive stresses 68 Development of residual compressive stresses……….. Thermal compatibility / Thermal expansion coefficient mismatching
- 69. This method involves strengthening glasses and ceramics by reinforcing them with a dispersed phase of a different material that is capable of hindering a crack from propagating through the material. 69 Interruption of crack propagation A. Methods of strengthening brittle materials
- 70. Tough crystalline material like alumina, leucite, lithia disilicate, magnesia is added in particulate form. Crack cannot propagate through alumina as easily as it propagates in the glass. E.g. Dicor Coefficient of thermal expansion between the particle and glass requires a close match. Interruption of crack propagation…… 70 Dispersion of crystalline phase
- 71. A crystalline material that is capable of undergoing a change of crystal structure when placed under stress is incorporated. E.g. partially stabilized zirconia which, at lower temperature, transforms into more stable & harder monoclinic phase with an increase in volume Refractive index of PSZ is higher than glass. 71 Transformation toughening Interruption of crack propagation……
- 72. Reducing Stress Raisers Stress Raisers are discontinuities in ceramic structures that cause stress concentration. E.g. Abrupt changes in shape or thickness in the ceramic, renders the restoration more prone to failure Creases or folds of the Platinum foil substrate in PJC, become embedded in the porcelain and leave behind notches (stress raisers). 72 B. Methods of designing components to minimize stress concentrations and tensile stresses
- 73. Minimizing Tensile Stress In Metal-Ceramic Crowns - The strong, yet ductile metal coping minimizes flexure of the porcelain structure in an attempt to overcome the associated tensile stress. Both, the Bonded Platinum foil technique and the Swaged Alloy foil technique are also based on this same concept. 73 Favorable occlusion in PJC helps to avoid tensile stress. B. Methods of designing components to minimize stress concentrations and tensile stresses
- 74. Esthetic Properties of Dental Ceramics The principal reason for the choice of porcelain as a restorative material is its esthetic qualities in matching the adjacent tooth structure in translucency, color and chroma. 74
- 75. Color production in natural teeth E Incident = E Scattered + E Reflected + E Absorbed +E Transmitted +E Fluoresced 75
- 76. 76 Incisal third Middle third Cervical/gingival third Enamel covering with little or no dentin underneath produces a wrap around effect which results in increased translucency in the incisal third and approximal areas. This region consists predominantly of dentin, hence the overlying enamel takes on some of the dentinal hue (yellow-orange) which is modified by the translucent blue grey enamel resulting in a composite colour. Enamel thins down towards the cervical line, hence the underlying dentinal hue results in a deep hue ranging from orange-yellow to often a distinct brown depending on the degree of calcification of dentin. Variations of Tooth Color
- 77. Dental porcelains are pigmented by the inclusion of oxides to provide desired shades. Specimens of each shade (collectively called a shade guide). Shade Guide 77 photo Disadvantages in using shade guides Tabs are much thicker Tabs are more translucent than teeth The necks of the shade tabs are made from a deeper hue and this region tends to distract the observers matching ability in the gingival third of the tab
- 78. Shade Matching Guidelines Remove all lipstick, heavy make-up, or large jewelry Use cool, color-corrected fluorescent lighting or sunlight near the window (during middle portion of the day ) If eyes seem fatigued to yellow, look at a blue napkin or blue wall to desensitize the eyes Select basic hue of tooth by matching the shade of patient’s canine (most highly chromatic tooth) Color matching should be done under two or more different light sources 78
- 79. Metal Ceramics Most widely used prosthesis system in fixed prosthodontics Mechanical properties Esthetic properties Metal Ceramic 79
- 80. Cross section of metal-ceramic crown 80 Parts of Ceramic Crown
- 81. Technical procedures in Metal –ceramic restorations Casting Heat degassing treatment Finishing Sandblasting Condensation of porcelain 81
- 82. Both the metal and ceramics must have coefficients of thermal contraction that are closely matched such that the metal has a slightly higher value. High proportional limit, high modulus of elasticity (to reduce stress on the porcelain) High fusion temperature (more than porcelain) Should exhibit minimal creep during firing of the porcelain 82 Ideal Requirements for Metals & Ceramic
- 83. Possess adequate mechanical strength for multiple splinting and bridge work. The surface metal oxide should not discolor the porcelain or interfere with glass formation. Biocompatible Chemically stable (high corrosion resistance) Ability to wet & bond metal surface 83 Ideal Requirements for Metals & Ceramic
- 84. 84 • High Noble alloys • Noble alloys • Base-metal alloys Types of Metal Ceramic Systems Cast metal ceramic alloys Foil Copings Bonded platinum foil coping Swaged gold foil coping
- 85. High Noble alloys Metal-ceramic alloys containing > 40 wt% gold and at least 60 wt% of noble metals (Gold, Platinum and palladium and/or the other noble metals) Platinum - hardens the gold Palladium - lowers the coefficient of thermal expansion Melting temperature : 1000oC-1150oC. 85
- 86. Noble alloys According to the ADA classification of 1984,noble alloys contain at least 25 wt% of the noble metals, but not necessarily contain any gold. Pd reduces tarnishing effect of Ag & Cu. Melting temperature : 1000oC-1250oC. 86 Cr or Ti alloys : Ni-Cr-Mo-Be, Ni-Cr-Mo, Co-Cr-Mo, Ti-Al-V. Superior mechanical properties. Melting temperature : 1300oC or more. Base-metal alloys
- 87. Advantages of Base Metal Alloys Higher hardness and elastic modulus (stiffness) values, permit the fabrication of thinner copings (upto 0.1mm) and thus its use in long span FPD’s. More sag - resistant at elevated temperatures. Substantial cost difference between base - metal and noble metal alloys. The intrinsic value of the component elements is significantly lesser than that of noble-metal alloys. 87
- 88. Limiting Features Higher solidification shrinkage requires special compensatory procedures to obtain acceptable fitting. Potential for porcelain delamination due to separation of poorly adherent oxide layer from the metal substrate. Potential toxicity of Beryllium and allergic potential of Nickel. 88 Poor resistance to tarnish and corrosion of nickel containing alloys. Chair side-grinding and polishing requires more chair-side time and the use of high-speed equipment due to the high hardness/strength.
- 89. Non - Cast Metal - Ceramic Systems Non-Cast Metal-Ceramic Systems are an advancement in the fabrication of metal-ceramic restorations, which permits the fabrication of a metal-ceramic restoration without waxing, investing or casting. It was introduced by Dr. Itzhak Shoher and Aaron Whiteman. 89
- 90. Bonding aluminous porcelain to platinum foil. Platinum foil is electroplated with tin & then oxidized in a furnace (degassing) Thicker cast metal coping is replaced by thinner platinum foil :: allowing more space for porcelain :: improved esthetics 90 Bonded Platinum Foil Coping
- 91. Use of a golden foil is intended to warm the color of the crown and facilitate tooth color (Yellowish tinge). Laminated swaged gold alloy foil supplied in fluted shape Foil is swaged onto die & flame sintered to form a coping “Interfacial alloy” powder is applied & fired, & the coping is then veneered with porcelain 91 Swaged Gold Foil Coping Renaissance type Burnishing the margin Flame sintering
- 92. Captek System Bonding to porcelain is achieved by the formation of an intermediate layer of material such as Capbond metal-ceramic ‘bonder’. Two strips of highly malleable metal powder impregnated ‘wax’ are adapted to a refractory die. The first strip contains a gold, platinum and palladium alloy and the second is impregnated with all-gold. 92
- 93. Captek System The first strip is fired onto a refractory die at 10750 C for 11mins producing a rigid porous layer. Application and firing of the second strip is said to result in capillary infiltration of the spongiform network by the molten gold, resulting in a metal alloy framework with density similar to that of conventional castings. 93
- 94. Development of a durable bond and the thermal compatibility between the porcelain and the alloy are the primary requirements for the success of a metal - ceramic restoration. 94 Metal-Ceramic Bonding Metal-Ceramic Bonding Techniques Mechanical Chemical
- 95. Mechanical Method Bonding surface of cast metal is made rough using diamond & carbide burs, or sandblasting (with pure alumina). This results in increased surface area. Mechanical interlocking Pd – Ag alloys form no external oxides- hence mechanical bonding 95
- 96. Primary bonding mechanism Presence of adherent oxide layer is essential for good bond formation In precious metals, tin oxide and iridium oxide are responsible for bond formation In base metals, chromium oxide forms the bond. For a good bond, metal substructure should be free of contamination Metal is oxidized in a furnace at 950oC for 5 min. Chemical Method 96
- 97. Ceramic bonding on to metals by electrodeposition of metal castings and heating to form suitable metal oxides. Alloy coping is electrodeposited with a layer of pure gold & a subsequent short “flashing” deposition of tin Used with Co-Cr, Pa-Ag, stainless steel, high- & low-gold alloys 97 Electrodeposition
- 98. Electrodeposition Improved bonding due to improved wetting and reduced porosity at the porcelain metal interface Electrodeposited layer acts as a barrier between metal casting & porcelain to inhibit diffusion of atoms from metal into porcelain Gold colour of the oxide film enhances the vitality and esthetics of the porcelain Tin oxide aids in chemical bonding The deposited layer acts as buffer zone to absorb stresses caused by differences in Coe. of thermal expansions 98 Advantages
- 99. Advantages of Metal-Ceramic High strength & durability High fracture resistance Permanent esthetic quality compared to acrylic veneer No staining along the interface between veneer & metal Adequate marginal fit 99 Flexure strains produced in long span bridges may fracture ceramics Slightly poor esthetics Darker margins near the gingiva Disadvantages
- 100. 1.Metal – Porcelain Seen when metal surface is devoid of oxides or due to porous and contaminated metal surface. 2.Metal oxide-Porcelain Oxide layer remains firmly attached to metal, seen mostly in base metal alloys. 3.Metal –metal oxide Metal oxide remains attached to porcelain, seen in base metal alloys due to overproduction of chromium and nickel oxides. 100 Bond Failures O’ Brien (1977)
- 101. Bond Failures 4.Metal oxide –metal oxide Fracture within the metal oxide. Results from overproduction of oxide causing sandwich effect between metal and porcelain. 5.Cohesive within metal Common in bridges where joint area breaks, rare in single crowns. 6.Cohesive within porcelain Tensile failure within porcelain. 101
- 102. Interfacial bond failure occurs primarily at three sites Along the interfacial region between opaque porcelain and the interaction zone. Within the interaction zone. Along the interfacial region between the metal and the interaction zone. 102
- 103. Porcelain Teeth High-fusing or medium-fusing porcelains Manufactured by packing two or more porcelains of differing translucencies for each tooth into split metal moulds & then fired at high temperature Mechanical interlocking to denture base Anterior teeth : projecting metal pins Posterior teeth : diatoric holes 103
- 104. More natural looking than acrylic teeth Excellent biocompatibility More resistant to wear than natural teeth Brittle Clicking sound on contact with opposing teeth Require greater inter-ridge distance (cannot be ground like acrylic teeth without destroying diatoric channels) Higher density- increases the weight Opposing natural teeth wear Disadvantages Advantages Porcelain Teeth…..
- 105. 105 All- Ceramic Restoration Excellent esthetics as all its thickness is for the porcelain. Earlier made up of traditional low-fusing porcelain fired onto a thin platinum foil. – PJC To improve its strength – use of a strong core of ceramics underneath the traditional porcelain. Core ceramics include: Aluminious based ceramic Magnesia based ceramic Glass infiltrated aluminous ceramic
- 106. Aluminous porcelains (Hi-Ceram) Mclean and Hughes - 1965 Contains 40-50% alumina Strengthens ceramic by interruption of crack propagation Used to construct core of PJC over which conventional enamel and body porcelain are condensed Mc lean 1979 Five year failure rate 2% for anteriors 15% for posteriors Seiber et al 1981: light reflection better than porcelain fused to metal
- 107. Aluminous porcelains…. More esthetic than PFM Strength twice that of conventional porcelain Requires less removal of tooth structure Disadvantages Advantages Inadequate strength to be used on posterior tooth More sintering shrinkage
- 108. Used in place of aluminous core porcelains High Coe. of thermal expansion (14.5 x10-6) Magnesia core porcelains
- 109. A glass ceramic material that can be casted using the lost wax process 1968 Mc Culloch Castable Ceramics Di-Cor Cerestore IPS Empress New types Cera pearl Optimal pressable ceramic Olympus castable ceramics
- 110. Castable glass ceramic It is formed in to desired shape as a glass then subjected to heat treatment to induce devitrification of glass Known as ceramming The crystalline particles thus formed interrupts crack propagation & improves strength and toughness The crown is cast at 1380oc Final shading is achieved with external staining
- 111. Dicor Dicor by Corning glass works excellent esthetics because of the “chameleon” effect. contains about 55 vol% of tetrasilicic fluormica crystals Increased strength, thermal shock resistance, decreased translucency Dicor MGC is a higher quality product that is crystallized by the manufacturer and provided as CAD – CAM blanks or ingots Low tensile strength Inability to be colored internally
- 112. Dicor ceramic crown
- 113. Wax pattern Spruing Investing Burnout Divesting Cast glass coping Ceramming 1750 for 12hr 450 for 12 hr Centrifugal casting 2600 f
- 114. Ceramming Ceramming oven Crystallised glass coping Conventional porcelain application & Firing Finished crown Cerramming done from room temparature- 1900 f for 1½ hrs and sustained for 6hrs inorder to form tetra silicic flouro mica crystals
- 115. Castable glass ceramic In another type ,ceramming produced hydrxyapatite crystals rather than mica as in dicor Coors porcelain company produced Cerestore Cerestore contains 70% alumina and is partially crystallized as alpha- Al203
- 116. All ceramic crowns without a core contains up to 45 vol% tetragonal leucite. The greater leucite content leads to a higher modulus of rupture, compressive strength and high thermal contraction of coefficient. This large thermal contraction causes mismatch between leucite and the glassy matrix results in the development of tangential compressive stresses in the glass around the leucite crystals when cooled, these stresses can act as crack deflectors and contribute to increase the resistance to crack propagation. Leucite reinforced ceramic (Optec HSP)
- 117. Advantages High flexural strength Excellent esthetics Disadvantages Abrasion against natural teeth is higher than that of conventional feldspathic porcelain. Poor marginal fit due to sintering shrinkage Requires special equipment for fabrication Indications Inlays Veneers Low stress crowns and veneers Leucite reinforced ceramic (Optec HSP)
- 118. IPS-EMPRESS (Pressable Ceramic) Hot pressed ceramics Leucite reinforced K2O – Al2O3 – 4 SiO2 Lithium Disilicate reinforced SiO2 – LiO2 – P2O5 – ZrO2 2 types IPS Empress IPS Empress 2
- 119. IPS Empress Developed by Wohlwend at the dental institute, Zurich University, 1991 Type of leucite reinforced pressable ceramics contains about 35 vol% leucite, available in pre-ceramed cylinders Crowns are formed using the lost-wax process and hotpressing leucite reinforced material into the mold using special furnace
- 120. Wax preparation is made and placed on a specially designed cylindrical crucible former and invested using a phosphate bonded investment, the mold is heated in a burnout furnace to 8500C. Wax pattern Investing Burn out 8500 C IPS Empress
- 121. The pre- ceramed cylinder is heated to 1100oc at which it is plastisized, injected under pressure at high temperature in to the mould for complete filling of investment cavity Injected under pressure at high temperature for 45 minutes into a mold to produce ceramic substructure. Ceramic ingot & Al plunger Pressing under vaccum 11500C Sprue removal 26 min hold IPS Empress
- 122. The crown is formed in dentin shades over which enamel layer is added The crown can be finished by two techniques staining, glazing or by layering, involving veneering porcelain. 122 IPS Empress Edward B Goldin 2005 compared leucite IPS Empress with PFM Mean marginal discrepancy 94 + 41 PFM 81 +25 IPS Clinical survival : Deniz G in 2002 95% survival 2-4 years Marginal adaptation : Shearer et al in 1996 : better marginal adaptation with hot pressed ceramics than aluminous core material.
- 123. Indications Anterior crowns Inlays Laminate veneers Post and cores IPS Empress Contraindications Clinical crown length of the tooth is exceptionally short tooth, reduction would compromise resistance and retention of the preparation. Parafunctional habit Advantages: High flexural strength (126-165 Mpa ) No shrinkage after pressing Excellent fit and esthetics Stability of shape Disadvantages: Limited to single tooth restoration Potential to fracture in posterior areas
- 124. IPS Empress
- 125. PROPERTIES Flexural Strength - 120 Mpa initially and 182 Mpa after heat treatment. Fracture toughness - 1.3 Mpa. Abrasion behaviour and Translucency -similar to that of natural teeth Solubility - <200mg/cm2 Pressing temperature - 11800 C Application of the - 9100 C sintered glass ceramic IPS Empress
- 126. IPS Empress 2 To extend the use of resin-bonded ceramic restorations and use them for bridge construction, a glass ceramic lithium based system has been developed. The framework is fabricated with lost-wax and heat-pressure technique IPS Empress 2 has a core of lithia disilicate crystals in a glass matrix and veneering ceramic contains apatite crystals which causes light scattering similar to that of enamel The core microstructure of the two is different which is responsible for slight decrease in translucency of IPS Empress 2 Lithium Disilicate reinforced
- 127. Full contouring Cut back Sprued pattern Investing Ingot pressing IPS Empress 2
- 128. The needle-like crystals cause cracks to deflect, thus the propagation of cracks through this material is arrested by the lithium disilicate crystals, providing a substantial increase in the flexural strength. IPS Empress 2
- 129. INDICATIONS Three unit bridges for the anterior and posterior regions upto the first premolar Crowns in anterior and posterior regions CONTRAINDICATION Short crown length Parafunctional habits IPS Empress 2
- 130. In-ceram A process used to form green ceramic shape by applying a slurry of ceramic particles and water or a special liquid to a porous substrate such as a die material, there by allowing capillary action to remove water and densify the mass of deposited particles Flexural strength 350 MPa 500 MPa 700 MPa In-ceram Alumina In-ceram Spinell In-ceram Zirconia ( Slip casting technique ) Saadoun 1989
- 131. Glass infiltrated alumina core porcelain- Inceram Relies on “slip casting” to produce high strength core Fine sized alumina core with improved strength The core is fired for 10hrs at 1100oc in a special furnace Glass is infiltrated in to core frame work over 4 to 5 hrs at 1120oc by capillary action, enhancing colour and strength
- 132. Glass infiltrated alumina core porcelain- INCERAM(ICA) The core of ICA consists of 70 wt% alumina infiltrated with 30wt% sodium lanthanum glass. Advantages Four times more strength than other ceramics Enhanced marginal adaptation Disadvantages Poor esthetics Complex procedure Cost Indications Anterior crowns and bridges Posterior crowns
- 133. Cross section of an INCERAM crown
- 134. Al2O3 slip Glass infiltration Vita Inceramat3 Giordono 1995 : Al2O3 Core glass infiltrated Ceramic > Strength than Hi-Ceram, Di-Cor & Feldspathic Porcelain Vaccumat 4000 Premium
- 135. Duplication In-Ceram refractory dies In-Ceram application Al2O3 slip 10 hrs 1120 c- 2hrs vita inceramat Working model Glass infiltration 4hrs 1100c Shrinkage of dies
- 136. Application of body and incisal porcelain Postoperative veiw of In-Ceram crowns Finished In- Ceram copings (Air abraded) Finished crowns Preoperative veiw Probster et al : Strength of In-Ceram > IPS Empress < PFM
- 137. Glass infiltrated spinell core (Inceram Spinell) (ICS) Offshoot of inceram It uses MgAl2O4 More translucent and so more esthetic Strength is low Inceram – Zirconia (ICZ) Has a core of 30 wt % zirconia and 70 wt % alumina Strongest and toughest of all three core ceramics Its use is limited to posterior crowns and FPDs because of its high level of core opacity
- 138. CAD-CAM Ceramics COMPUTER AIDED DESIGN-COMPUTER AIDED MANUFACTURING Examples - Cerec (Sirona), Sirona InLab, Everest (Kavo), Cercon (Dentsply), Lava (3M ESPE), Zeno (Weiland), 5- tec (Zirkonzahn), etc The CAD-CAM system consists of 5 essentials Scanner or digitizer – Virtual impression Computer – Virtual design (CAD) Milling station – Produces the restoration or framework Ceramic blanks – Raw material for the restoration Furnace – For post-sintering, ceramming etc.
- 139. 139
- 140. 140
- 141. 141
- 142. 142
- 143. 143
- 144. 144
- 145. 145
- 146. 146
- 147. 147
- 148. Copy milled ceramics A new system (Celay by Mikron Technologies, Switzerland) uses a copy milling technique to produce ceramic cores and substructures for bridges A pattern of coping is prepared directly or indirectly with special blue resin based composite A tracing tool passes over the pattern and guides a milling tool which grinds a copy of the pattern from a block of ceramic (Inceram or Inceram spinell) It is then infiltrated with glass and veneered with porcelain and fired to complete the restoration
- 149. Introduced in 1992, by Dr. Stefan Eeidenbaez, Zurich Uses copy-milling technique to manufacture ceramic inlays or onlays from resin analogs It is fine grained feldspathic porcelain that is said to reduce the wear of antagonist tooth structure Mechanical device based on pantographic tracing of a resin inlay or onlay fabricated directly onto the prepared tooth or onto the master die Material used is a ceramic blank available in different shades, contains sanidine as the major crystalline phase within a glassy matrix. Celay
- 150. Celay Scanning of the prepared cavity is done with a 3-D scanner, restoration is designed from the image shown on the computer screen by using a series of icons or symbols Can electronically design the restoration by moving a cursor along the limits of the preparation, thereby defining its boundaries Design phase usually takes from 2 to 8 minutes After data have supplied, the computer selects the size of ceramic block to be used in the milling process Diamond wheel is driven by the electric motor, which generally takes 4 to 7 minutes to complete the procedure
- 151. CELAY It is then infiltrated with glass and veneered with porcelain and fired to complete the restoration Cementation involves etching the tooth with a 37 % solution of phosphoric acid for 20 seconds, tooth is then washed and dried and a bonding agent is applied, ceramic restoration is etched on its undersurface, outside the mouth Dual cure microfill composite resin luting agent is used to bond the inlay, onlay or veneer, after photocuring the occlusal anatomy can be created, accomplished intraoraly with fine-particle diamonds.
- 152. CELAY ADVANTAGES Single appointment Bonded restoration for strength. Reduced marginal gap Hardness similar to enamel Less fracture of the inlay because it is milled from a solid Homogeneous block Excellent polishing characteristics, esthetics Preparation, fabrication, Cementation in 1 to11/2 hours.
- 153. Procera Introduced in 1994 Embraces the concept of CAD/CAM to fabricate dental restorations Available as Procera laminate Procera crowns Procera Bridge Procera Implant Bridge This crown is composed of a densely sintered high-purity aluminum oxide coping that as combined with the low-fusing Allceram veneering porcelain Content of aluminum oxide in these coping is 99.9% and the strength for this ceramic material is highest among all-ceramic restoration
- 154. PROCERA PREPARATION Die is prepared from impression, scanned at a local laboratory, which is saved as file in computer and send to laboratory in Sweden, where coping is prepared Coping is produced by a special process, which involves sintering 99.5% pure alumina at 1600–1700°C,fully densified coping is then returned to the dental laboratory for building in the crown’s aesthetics using compatible feldspathic glasses, turnaround time is approximately 24 hours
- 155. PROCERA ADVANTAGES Biocompatibility -Aluminum oxide coping material does not show any leakage or dissolution of aluminum at any of the pH levels Occulsal surface will not damage the opposing natural tooth Translucency-Procera coping is translucent, thus will not allow any staining of the underlying dentin High strengthFlexural strength 700 mpa. DISADVANTAGES Very few laboratories offer this system INDICATIONS Used in metal sensitive patients
- 156. Procera Alltitan In this technique titanium core is used, the external contours of the individual titanium cores for bridges are milled and graphite rods create the fitting surface by the spark erosion process. Individual components of the bridge are then welded by laser before the addition of special porcelains to layer the surface to the full contour
- 157. CEREC Introduced in 1991, feldspathic porcelain of high strength and fine grain size is used. Used in cases of inlays, onlays, partial crowns, crowns (posterior & anterior), and veneers One of the most researched restorative systems on the market, with documented success rates of more than 90% after 10 years
- 158. Preparation Of CEREC A cast of the prepared teeth made using a specialized CAD-CAM- compatible stone that is sprayed with Quickcheck indicator spray After the die preparation, the die is loaded into the bridge holder of the CEREC inLab unit; the bridge holder is then placed in the machine for scanning. A digital image of the cast is displayed on the screen, In-Ceram block is inserted into the unit for automatic milling
- 159. CEREC After milling is complete, the fit of framework is tested on the die stone. Consistency of a coping is chalk-like, any necessary adjustments can be accomplished quickly and easily The proper amount and shade of glass required is applied The coping is placed in the In-Ceram furnace for infiltration.
- 160. CEREC Excess glass is removed by sandblasting the coping, luminary coat is applied for refraction of light. The appropriate shade of porcelain and modifiers are applied for a natural appearance Glaze is applied, and the restoration is now complete and ready for placement
- 161. LAVA Introduced in 2002, Lava uses a laser optical system to digitize information from multiple abutment margins The Lava All-Ceramic System comprises a CAD/CAM procedure for the fabrication of allceramic Crowns and Bridges for anterior and posterior applications. CAD software scan the die automatically finds the margin and suggests a pontic, the framework designed to be 20% larger to compensate for sintering shrinkage
- 162. LAVA After the cut dies of the preparation is made, the milling center will digitalize the model by using the optical scanner Lava Scan The restoration will then be virtually designed on the monitor using a CAD, the data is sent to Lava Form, a milling unit (CAM) The restoration is milled from a pre-sintered zirconia blank, which can be colored in 8 different shades and which is then sintered to its final density in the furnace The milling center returns the finished framework to the lab who will then veneer the framework with Lava Ceram and give it the final artistic finish.
- 163. LAVA ADVANTAGES With the classic color scheme, all tooth shades can be easily reproduced, special effect components and stains lead to a natural esthetic High level of biocompatibility Anterior and posterior crown and bridge
- 164. Conclusion The difference with & without Ceramics is self evident 164
- 165. Journal of Indian Prosthodontic society –oct.2002,vol.2 no.3 Notes on dental materials- V K Subbarao Basic dental materials- Manappallil The science and art of dental ceramics –Mc lean vol 2 References 165
- 166. References Anusavice : Philips’ Science of Dental Materials Xth & XIth Edn. Craig : Dental Materials : Properties & Manipulation VIth, VIIth & VIIIth Edn. J. F. McCabe : Applied Dental Material VIIth Edn. Jack Ferracane : Materials in Dentistry Principles & Application 166
- 167. Repair of Ceramic Restoration This can be done if the fracture is not too extensive. Repaired in the mouth using a resin composite in dry field.
- 168. Residual stresses in porcelain when coe. Of thermal expansion of porcelain is more than metal
- 169. Metal-Ceramic Bonding Fusion temperature of ceramics : 900oC-1000oC. Highly viscous liquid having large surface tension (365 dynes/cm). Angle of contact (130o) with alloy surface. Ceramic liquid does not wet & bond with metal surface. 169
Editor's Notes
- He suggested the use of jeweler’s enamel to fabricate artificial teeth.
- Ceramic jacket crown (CJC) - an all-ceramic crown without a supporting metal substrate that is made from a ceramic with a substantial crystal content (>50 vol%) from which its higher strength and/ or toughness is derived. These crowns are distinguished from porcelain jacket crowns that are made with a lower-strength core material, usually aluminous porcelain or feldspathic porcelain (see porcelain jacket crown). Porecelain jacket crown (PJC) - one of the first types of all-ceramic crown, made from a low-strength aluminous core porcelain and veneering porcelain (with matching thermal contraction coefficient) without the use of a supporting metal substrate except, in some instances, for a thin platinum foil (see ceramic jacket crown).
- Interestingly, some of these early designs were ahead of time. Their failure to gain widespread popularity could probably be attributed to the fact that prosthetic technique, antibiotic use, infection control, instrumentation, and impression materials had not yet advanced far enough. 1923 - Wain described fabrication of inlays and onlays using dental porcelain.
- The medium-fusing and high-fusing types are used for the production of denture teeth. The low-fusing and ultra – low fusing porcelains are used for crown and bridge construction. Some of the ultra-low fusing porcelains are used for titanium and titanium alloys because of their low-contraction coefficients that closely match those of the metals and because the low firing temperatures reduce the risk for growth of the metal oxide.
- Pg 16 Is a potassium-aluminum-silicate mineral with a large coefficient of thermal expansion ( 20-25x10o/ oC) compared to feldspathic glasses ( 10x10o/oC). It is an artificial crystal feldspathoid ( K2O.Al2O3.4siO2) formed by the incongruent melting (Incongruent melting is the process by which one material melts to forma liquid plus a different crystalline materials) of feldspar ( K2O.Al2O3. Al2O3-4siO2). In most dental porcelains, the leucite crystals are created by transforming feldspar crystals into glass and leucite crystals (precipitate) by a special heat treatment The larger the proportion of feldspar, greater the translucency and glass like appearance of the resultant ceramic material
- Thus, the major difference between industrial porcelain and dental porcelain was the lesser proportion of kaolin in denal kaolin.
- Silica -It is one of the most abundant elements on earth and can exist in many different forms such as crystalline materials like Quartz, Cristobalite, Tridymite and amorphous materials like Fused quartz, Agate Jasper and Onyx. Pure Quartz crystals (SiO2) are used for manufacturing dental porcelain. It is made by selecting uniformly light-coloured pieces of quartz free from traces of iron which are ground or ball milled to the finest grain size possible
- glass consisting of 3-dimensional network of silica with a very high fusion temperature Manufacturers employ glass modifiers to produce dental porcelains with different firing temperature such as high, medium and low fusing ceramics. Vitrification’ in ceramic terms is the development of a liquid phase by reaction of melting, which on cooling provides the glassy phase, resulting in a vitreous structure. However, a small amount of crystallization always occurs during glass formation, although the rate of crystal growth is very low. When a glass begins to crystallize, the process is called Devitrification. Alkalis such as soda (Na2o) and lime (CaO) lower the viscosity, and thus the glass transition temperature, considerably, by causing extensive disruption of SiO4 tetrahedrals are disrupted, the glass may crystallize or devitrify Devitrification may be seen when cloudiness develops in dental porcelain and this can be accentuated by repeated firing
- Addition of glass modifiers to reduce the softening point also decreases the viscosity, resulting in slump or pyroplastic flow; hence it is necessary to produce glasses with high viscosity as well as low firing temperature (This phenomenon is termed as boron anomaly).
- Although not an intentional addition, . It may also account for the occasional long-term failure of porcelain restorations after several years of service.
- The greatest colour problem encountered, with porcelain if not coloured is the slightly greenish hue exhibited by all glasses. In addition, some porcelains assume a greenish hue after firing, and this inherent greenness can be further accentuated by overfiring (over-vitrification). In order to dampen down this effect and produce life-like dentine and enamel colors; the basic dental porcelain frit must be colored Stain is a mixture of one or more pigmented metal oxides, and usually composed of a low fusing glass that when dispersed in an aqueous slurry or monomer medium applied to surface of porcelain or other specialized ceramic, dried or light cured, and fired will modify the shade of the ceramic-based restoration. It is more concentrated than a colour modifier and is generally used as a surface colorant or to provide enamel check lines, decalcification spots etc. in the body of a porcelain jacket crown. These stain products are also called as surface colorants or characterization porcelain.
- Stain is a mixture of one or more pigmented metal oxides, and usually composed of a low fusing glass that when dispersed in an aqueous slurry or monomer medium applied to surface of porcelain or other specialized ceramic, dried or light cured, and fired will modify the shade of the ceramic-based restoration. It is more concentrated than a colour modifier and is generally used as a surface colorant or to provide enamel check lines, decalcification spots etc. in the body of a porcelain jacket crown. These stain products are also called as surface colorants or characterization porcelain.
- Opacifiers are added to reduce the translucency.
- Pyrochemical reactions during manufacture of porcelain: When the ceramic raw materials are mixed together in a refractory crucible and heated to a temperature well above their ultimate maturing (fusion) temperature, a series of reaction occur. After the water), kaolin (binder) and feldspar (basic glass former) and partly combines them together. The process of blending, melting and quenching the glass components is termed ‘fritting’. The fused mass is then quenched in water. Binder – helps to hold the particles together, as the porcelain material is extremely fragile in the ‘green’ state. Types of binder used : Distilled water – most commonly used, especially for dentin / enamel porcelain, Prophylene glycol – used in alumina core build-up, Alcohol or formaldehyde based liquids – used for opaque core build up The process of blending, melting and quenching the glass components is termed ‘fritting’.
- Impression of the prepared tooth is recorded & a die is prepared in a refractory material. Porcelain powder is mixed with water or a special liquid to form a thick slurry, which is then applied over the platinum foil. Instruments recommended for building porcelain: Sable hairbrush Carving & Grooving Blades Mixing spatulas – Bone or agate (b) Stainless – steel twin bowl and sponge for cleaning brushes, and (c) Modified pliers for holding the restoration / casting from its internal aspect.
- . It is a 2-part process – Agitation of the particles & Removal of excess moisture. Mixing: Dry porcelain powder is mixed with the binder on a glass slab using bone or nylon spatula (or glass mixing rod) into a thick creamy mix, which can be carried in small increments with an instrument or brush
- Gravitational Whipping Condensing Aluminous core porcelain Since the strength of aluminous core porcelain depends on the condensation of the crystal / glass composite, it is thoroughly vibrated to ensure maximum density in the powder bed. A thick creamy mix is spread evenly over the platinum matrix. When the matrix is covered the die is vibrated with the serrated end of a Le-Cron Carver until all the moisture rises to the surface and can be absorbed with a tissue paper. Subsequent applications are performed similarily
- continuous vibrations at a frequency of 20,000 – 28,000HZ
- In Vaccum – fired porcelain when fired in a partial vaccum, the air or atmosphere is removed from the interstitial spaces before sealing of the surface occurs. However, all the air is not removed and the residual air becomes sphere-shaped under the influence of tension and increased furnace temperature. When air at normal atmosphere pressure is once again allowed to enter the furnance muffile, it exercises a strong compression effect on the dense surface skin, which hydraulically compresses low-pressure internal bubbles. This results in realatively dense pore-free porcelain because the remaining bubbles are few in number porcelain because the remaining bubbles are few in number and extremely small size. Limitations of vaccum firing – Large bubbles trapped due to poor condensation technique cannot be reduced in size to any significant degree and can be seen as blistering of the material. Advantages over air-fired porcelains: Improved esthetics
- the surface appearance of un-glazed porcelain is “bisque” or biscuit since this gives a fairly accurate picture of its surface texture
- Cohesion Incomplete
- Surface becomes smooth & glossy & its chemical durability is higher than over glaze due to high fusion temperature.
- Dental Porcelains on cooling will undergo differential contraction and surface microcracks will appear
- The smooth glossy surface resists the adherence of exogenous stains. In fact over a period of years, a porcelain restoration may develop a mismatch with adjacent teeth caused by changes in colour of the adjacent natural teeth with age. Degradation of dental ceramic generally occurs because of mechanical forces or chemical attack. The possible side effects of ceramics is their tendency to abrade opposing dental structures, the emission of radiation from radioactive components, the roughening of their surfaces by chemical attack with a corresponding increase in plaque retention and the release of potentially unsafe concentrations of elements as a result of abrasion and dissolution. The chemical durability of ceramics is excellent. With the exception of excessive exposure to acidulated phosphate fluoride (APF), Ammonium bifluorides, and Hydrofluoric acid (HF), there is little danger of surface degradation for all types of ceramics.
- Dimensional Stability-thermal expansion When different porcelain formulations are veneered together (all-ceramic) and over metal copings (metal – ceramics) ,cofficient of thermal expansion should be matched to prevent development of interfacial stresses leading to separation or fracture. Flexural strength – ability to resist fracture when loaded which is a combination of compressive and tensile strength.
- Brittleness is the relative inability of a material to sustain plastic deformation before fracture of the material occurs. Brittle materials have a tensile strength that is markedly lower than the corresponding compressive strength. Fracture toughness is a parameter that quantifies the resistance of a material to crack propagation. It is non-ductile; therefore failure occurs as brittle fracture begins in areas of tension due to concentration of stress around minor surface irregularities
- The largely covalent or ionic bonded structure of ceramics confers resistance to chemical degradation in the oral environment, however it also imparts brittleness While the theoretical strength of porcelain is dependent upon the silicon – oxygen bond, the practical strength is 10 to 1000 times less than the nominal strengths The brittle behavior of ceramics and their low tensile strengths compared with those predicted from bonds between atoms can be attributed to the phenomenon of stress concentration around surface flaws. When the theoretical strength of the material is exceeded at the tip of the notch, the bonds at the notch tip break and initiate crack formation. As the crack propagates through the material, the stress concentration is maintained at the crack tip until it meets another crack, pore or a crystalline particle. the materials can with stand a deformation of approximately 0.1% before fracture ), thus, cracks may propagate through a ceramic material at low average stress levels The moist environment of the oral cavity aggravates the low tensile strength of dental ceramics, by weakening the silicon-oxygen bond.
- Ceramics have good compressive strength but they are brittle and have low tensile strength.
- 1. Development of residual compressive stresses 2. Interruption of crack propagation through the material. Ion exchange – (Chemical tempering)
- Is one of the more sophisticated and effective methods of introducing residual compressive stresses into the surface of ceramics. Although a pronounced strengthening effect occurs, not all ceramics are amenable to ion exchange.
- Metals having higher coefficient of thermal expansion than ceramic and ceramic used along with it are heated & cooled together.
- When subjected to heat treatment, Mica crystals grow in situ which interrupt crack propagation.
- The energy that would allow crack to propagate is used for transformation of partially stabilized zirconia. they scatter light as it passes through porcelain producing an un-esthetic opacifying effect in the final restoration
- sharp angles In Porcelain Jacket Crowns (PJC’s): Several conditions that cause stress concentration in PJC’s are : Creases or folds of the Platinum foil substrate that become embedded in the porcelain and leave behind notches (stress raisers).
- (PJC’s) stresses near the interior surface of the crown A metal coping is used as the foundation of the restoration to which the porcelain is fused.
- The bulk of tooth structure is comprised of 2 layers of calcified tissues; enamel and dentin, surrounding a central core or pulp chamber. As the light ray strikes the tooth surface, part of it is reflected, and the remainder penetrates the enamel and is scattered. Dentin lying below the enamel is more opaque than enamel and reflects light. Any light reaching the dentin is either absorbed or reflected to be again scattered within the enamel. If dentin is not present, as in the tip of an incior, some of the light ray may be transmitted and aborbed in the oral cavity. As a result, the incisal area may appear to be more translucent than that towards the gingival area. Thus, because the law of energy conservation must apply, the following relationship shows the four energy components that are derived from the energy (E) of the incident light. Although some of the absorbed light may be converted into heat, some may be transmitted back to the eye as fluorescent energy. When the ultra violet ray of daylight or of night club lighting contact teeth or restorations, some of the radiant energy is converted into light of one or more colours, for example, red, orange and yellow.
- is also apparent in different regions of the tooth such as the incisal, middle and cervical/gingival third.
- Therefore, the appearance of the teeth may vary according to whether they are viewed in direct sunlight, reflected daylight, tungsten light or fluorescent light. This phenomenon is called metamerism It is impossible to imitate such an optical system perfectly. The dentist and/or laboratory technician can however, reproduce the aesthetic characteristics sufficiently such that the difference is consicuous only to the trained eye. tabs are much thicker than the thickness of ceramic that is used for dental crown or veneers. Shade guide tabs are more translucent than teeth and ceramic crowns that are packed by a nontranslucent dentin substructure (metal coping). While much of the incident light is transmitted through a tab, most the incident light on a crown is reflected back except at the incisal edges and at inciso-proximal areas. The necks of the shade tabs are made from a deeper hue ( i.e., higher chroma) and this region tends to distract the observers matching ability in the gingival third of the tab (to avoid this situation, some clinicians grind away the neck area of a set of tabs).
- Pumice the teeth if external stains are present Match the color of teeth with shade guide under illumination of northern light from a blue sky
- If a stronger material is used as an inner core of a PJC, then cracks can develop only when the core is deformed or broken; assuming that the porcelain is firmly bonded to the reinforcing substrate. This concept is applied in porcelain-fused-to-metal (PFM) restorations, where porcelain is fused directly to a metal coping (cast-alloy substructure) serving as a metallic substrate that fits the prepared tooth. These restorations combine the strength and accuracy of cast metal with the esthetics of porcelain.
- Metal alloy substructure is cast using a phosphate-bonded investment. - Heat treatment is done to produce a surface oxide layer and ensure a clean meta surface for bonding.-at 980oc - Ceramic bonded stones or sintered diamonds are used for further cleaning and surface finishing. - Final sandblasting with high-purity alumina abrasive ensures that the porcelain is bonded to a clean and mechanically retentive surface. Opaque porcelain (0.3mm) layer over the metal. Opaque porcelain is followed by body and translucent enamel porcelains before a final glaze is obtained (as with the PJC).
- coefficients of thermal contraction – low – 0.5X 10 -6 /0c to avoid residual stresses in porcelain if high weaken the porcelain and the bond Creep/ sag : When the temperature approaches 980C (1800F), high-temperature flow termed creep or sag of some noble alloys occurs. This can be reduced by proper metal composition so that a disprersion strengthening effect occurs at high temperatures, resulting in precipitating of a second phase that can harder or strengthen the alloy. High temp metal deformation- sag
- The metal or metal oxide should not corrode or produce toxic effects in surrounding tissues.
- According to noble metal content ,metal alloys are broadly classified by the ADA (1984) into 3 major categories
- Coefficient of thermal expansion {CTE} is inversely propotional to melting point of metals and melting range of alloys).
- The noble classification generally refers to all Pd-based alloys that contain : 54 - 88 wt% Pd for metal-ceramic restorations. 25 wt% Pd for all metal/ resin-veneer restorations.
- Biologic hazards of base-metal alloy: Nickel : Allergic potential : Contact dermatitis reaction in certain individuals. Precautions should be taken to prevent aspiration of air-containing nickel-dust produced during grinding operations. Beryllium : Added to reduce the fusion temperature. Toxic effects are mainly due to inhalation of vapor during melting and grinding procedures. Hence, adequate ventilation is essential.
- Bonding aluminous porcelain to metal using tin oxide coatings on platinum foil.
- fluted shape (umbrella shaped reminiscent of a miniature coffee filter).
- Capbond metal-ceramic ‘bonder’ (bonding agent) for the Captek foil crown system Method : The first strip is fired onto a refractory die at 1075 C for 11mins producing a rigid porous layer. Application and firing of the second strip is said to result in capillary infiltration of the spongiform network by the molten gold, resulting in a metal alloy framework with density similar to that of conventional castings. Due to the gold colouration, a minimal thickness of opaque porcelain is required prior to application of translucent porcelains.
- Capbond metal-ceramic ‘bonder’ (bonding agent) for the Captek foil crown system Method : The first strip is fired onto a refractory die at 1075 C for 11mins producing a rigid porous layer. Application and firing of the second strip is said to result in capillary infiltration of the spongiform network by the molten gold, resulting in a metal alloy framework with density similar to that of conventional castings. Due to the gold colouration, a minimal thickness of opaque porcelain is required prior to application of translucent porcelains.
- Metal-Ceramic bonding is a complex mechanism. Ceramic materials heated to the proper high temperatures bond to the metal framework by mutual diffusion of metal ions from different elements into the ceremic and elements of the ceremic into the alloy.
- Coefficient of thermal expansion of alloys is decreased by adding Pt or Pd to HN or N alloys to 13.5-14.0 x 10-6/oC; & that in ceramics is increased by Na+, K+ or Ca++ ions or forming more leucite, to 13.0-13.5 x 10-6/oC. Slight mismatching of 0.5 x 10-6/oC helps bonding by mechanical interlocking.
- Fracture because of low ductility
- Anterior teeth have projecting pins that engages the denture base Posterior teeth have diatoric holes on the underside in to which the denture resin flows
- Core ceramic cannot be used for the entire restoration as it lacks esthetic qualities. Hence special surface ceramics is used which should also match the coefficient of thermal expansion of core and special surface ceramic.
- more
- Non porous, homogenous, microstructure with uniform crystal size which is derived from the controlled growth of crystals within an amorphous matrix of glass.
- First commercially available castable glass material for dental use was
- Higher strength but not as strong than those built on a core
- More translucent than aluminous core crowns or glass infiltrated ceramic- Excellent aesthetics
- The high strength of lithium disilicate glass ceramics creates the possibility of not only producing anterior and posterior crowns, but also all-ceramic bridges.
- Due to the translucency of the core, the final restoration is more aesthetic
- With the CEREC inlab System, all-ceramic are fabricated with CAD/CAM, while maintaining the same preparation and placement techniques currently employ for PFMs