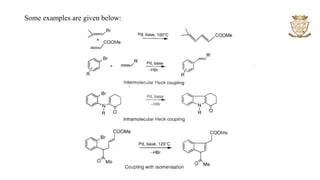
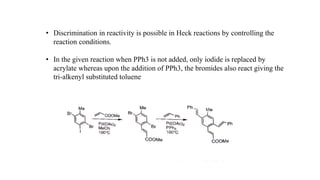
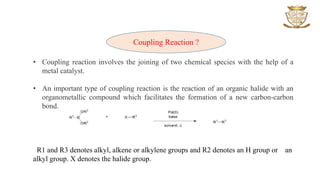
The document discusses various types of coupling reactions facilitated by metal catalysts, including Hiyama, Kumada, and Heck coupling. Key aspects include the mechanisms, advantages, and disadvantages of each method, with a focus on the formation of carbon-carbon bonds through reactions of organic halides with organometallic compounds. It emphasizes the importance of reaction conditions and the role of specific catalysts to achieve desired outcomes in synthesis.


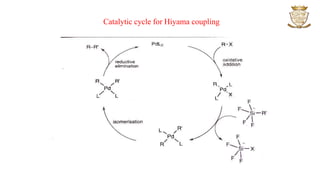
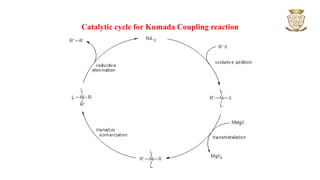
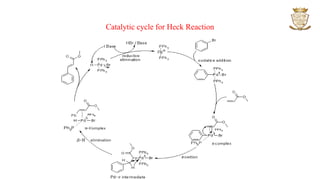
![• The reaction proceeds through
oxidative addition,
transmetallation,
cis-trans isomerization and
reductive elimination.
• The purpose of the fluoride ion is to activate the conversion of silicon compound RSiR3 to
a pentacoordinate [RSiR3F]- intermediate which is more amiable to transmetallation.
• Reactions in which the fluoride ion is replaced by a strong base have also been reported.](https://image.slidesharecdn.com/couplingreactions-230318065458-1a008005/85/Coupling-Reactions-6-320.jpg)