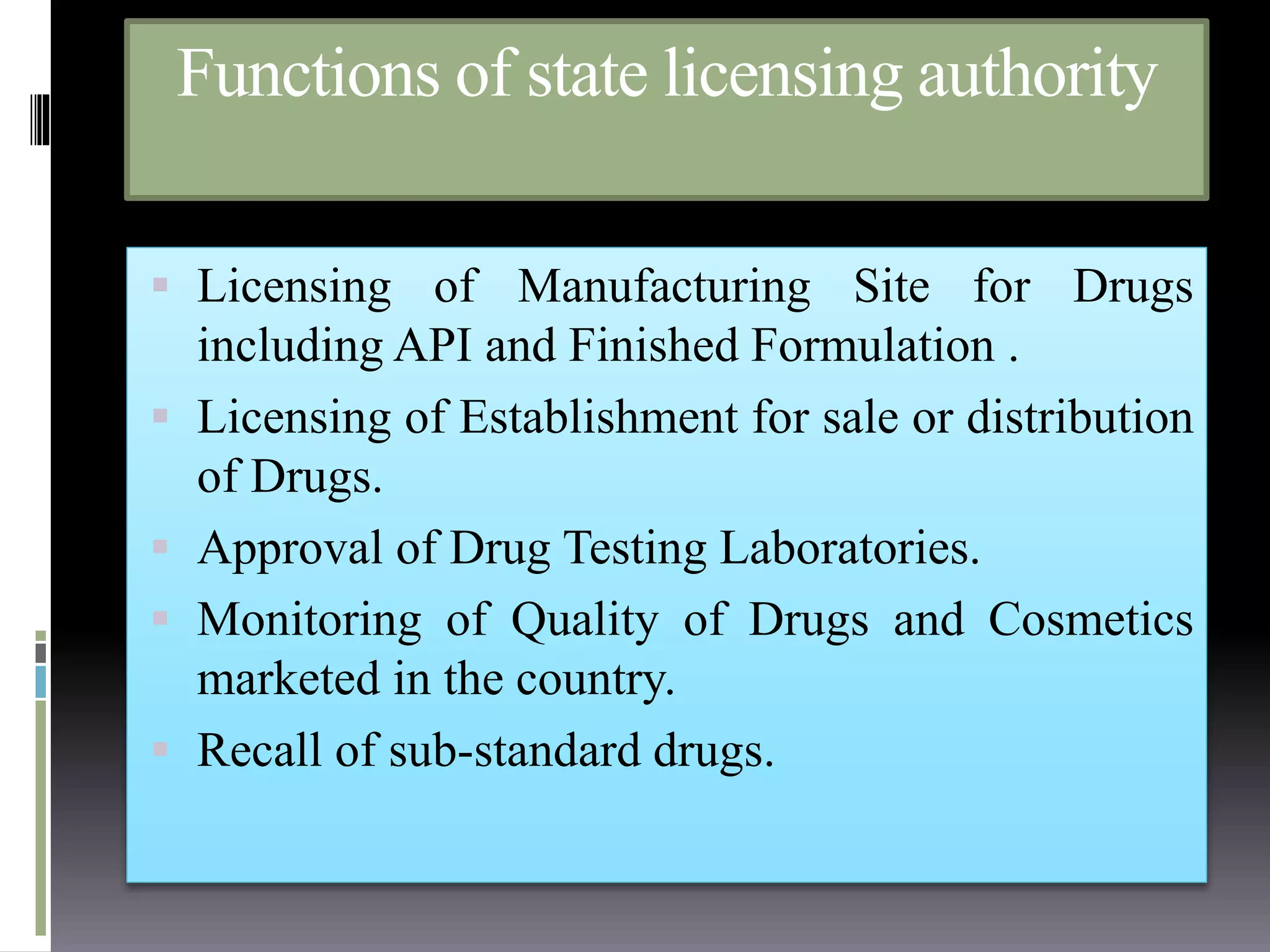
This document outlines the regulatory requirements and approval procedures for drugs, cosmetics, medical devices, biological products, herbal medicines, and foods/nutraceuticals in India. It discusses the key regulatory bodies like the Central Drugs Standard Control Organization (CDSCO) and the application processes. For drugs, the new drug approval procedure is described involving applying to the DCGI and undergoing clinical trials and reviews. For other products, the document explains the application forms and documents required for approval from bodies like FSSAI.

















![References
Patel j, Parikh k, shah d. NEW DRUG APPROVAL PROCEDURE IN
INDIA | PharmaTutor [Internet]. Pharmatutor.org. 2018 [cited 08
december 2018]. Available from:
https://www.pharmatutor.org/articles/new-drug-approval-
procedure-india.
Welankiwar A. REVIEW: REGULATORY PROVISIONS
REGARDING COSMETICS IN INDIA | PharmaTutor [Internet].
Pharmatutor.org. 2018 [cited 08 december 2018]. Available from:
https://www.pharmatutor.org/articles/review-regulatory-
provisions-regarding-cosmetics-in-india.
India Medical Device Registration - CDSCO Approval [Internet].
Pacific Bridge Medical. 2018 [cited 9 december 2018]. Available
from: https://www.pacificbridgemedical.com/regulatory-
services/medical-device/product-registration/india/
Home | Ministry of AYUSH | GOI [Internet]. Ayush.gov.in. 2018
[cited 9 December 2018]. Available from: http://ayush.gov.in/](https://image.slidesharecdn.com/presentation1-181211052213/75/regulatory-requirnment-and-approval-procedure-for-drugs-and-cosmetics-medical-devices-biologicals-and-herbals-and-food-nutraceuticals-in-india-19-2048.jpg)
![Reference continued…
[Internet]. Cdsco.gov.in. 2018 [cited 09 december 2018]. Available from:
https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/Pdf-
documents/medical-device/Guidance1.pdf.
. Us A, Mission F, Us C, Subscription M, Archive M, Policy P et al. Food
Regulations—What is the Current Scenario in India? - Food Quality &
Safety [Internet]. Food Quality & Safety. 2018 [cited 09 december 2018].
Available from: https://www.foodqualityandsafety.com/article/food-
regulations-what-is-the-current-scenario-in-india-2/.
home [Internet]. Cdsco.gov.in. 2018 [cited 09 december 2018]. Available
from: https://cdsco.gov.in/opencms/opencms/en/Home/.
[Internet]. Cdsco.nic.in. 2018 [cited 09 december 2018]. Available from:
http://www.cdsco.nic.in/writereaddata/statefunction.pdf.
DefinedTerm: State Licensing Authority [Internet]. DefinedTerm. 2018
[cited 09 december 2018]. Available from:
https://definedterm.com/state_licensing_authority.](https://image.slidesharecdn.com/presentation1-181211052213/75/regulatory-requirnment-and-approval-procedure-for-drugs-and-cosmetics-medical-devices-biologicals-and-herbals-and-food-nutraceuticals-in-india-20-2048.jpg)
