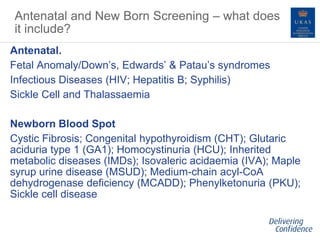
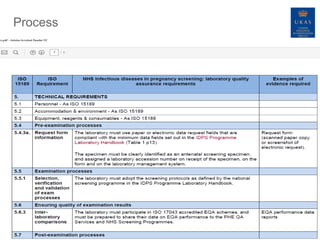
This document discusses the process of incorporating Public Health England's antenatal and newborn screening standards into UKAS accreditation to ISO 15189 for laboratories performing infectious diseases in pregnancy screening. The objectives are to reduce assessment burden, improve communication and patient safety through a coordinated accreditation and quality assurance process. PHE screening requirements have been mapped to ISO 15189. Laboratories must meet both PHE and ISO standards to receive and maintain accreditation, with information sharing between UKAS and PHE's quality assurance program. The process aims to strengthen oversight of screening laboratories while minimizing duplicate efforts.