Atomic Structure.pptx
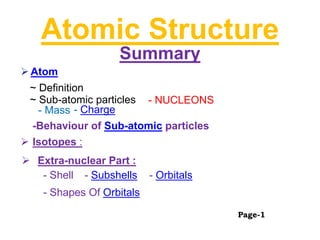
- 1. Atomic Structure Summary Atom ~ Sub-atomic particles - Mass - Charge - NUCLEONS Isotopes : -Behaviour of Sub-atomic particles Extra-nuclear Part : - Shell - Subshells - Orbitals - Shapes Of Orbitals ~ Definition Page-1
- 2. Electronic Configuration : o s p d f Notation o Noble Gas Core o Electrons-in-Boxes ~ Transition Elements [ 18Ar ] 4sx 3dy Ionization Energy : - Definitions - Types - Factors - Atomic Radius - Nuclear Charge - Shielding / Screening - Questions : -Trend 1, 2 - Sudden Change - Graphs Rules: Energy Rule Degenerated Orbital Rule Orbital Rule Page-2 ~ Ions Configuration
- 3. Atomic Structure Atom that can take part in a chemical reaction ~ Two parts Nucleus Extra nuclear part ~ Protons & Neutrons ~ Electrons ] NUCLEONS 23 𝑵𝒂11 Protons = 11 , Electrons = 11 , Neutrons = 12 Nucleons = 23 Symbol Relative Mass Relative Charge 𝐩𝟏+ 𝒏𝟎 𝐞𝟏− ~ Smallest part of element & Three Sub-atomic particles 1 2000 or 1 1860 or 1 1840 1- 1 1+ 1 0 Atomic Structure Lecture- 1 AS Level Chemistry
- 4. ~ Charge of any material depends on : Protons & Electrons o Three types of Charges Neutral Cationic Anionic ~ Protons equal to Electrons ~ Protons greater than Electrons ~ Protons less than Electrons ] Oxidation ] Reduction ~ Chemical Changes , control charge , ~ depend on Electrons . ~ Mass of an Atom depends on : Protons & Neutrons Nucleus ~ Physical Changes , depend on Neutrons ( Nucleus ) X Only Atomic Structure Lecture- 1 AS Level Chemistry
- 5. Isotopes : Definitions : ~ Atoms of the same element ~ having same atomic number but different mass number. 2 ~ Atoms of the same element ~ having same electrons & protons but different neutrons. 1 1 1 1 In terms of Sub- atomic particles Same Chemical properties Different Physical properties ~ same Electrons ( Valence ) ~ different Neutrons 1H1 2H1 3H1 X H2O D2O Mass Low High B.P. Low High 2 H2O + 2 Na 2 NaOH + H2 2 D2O + 2Na 2 NaOD + D2 1 Atomic Structure Lecture- 2 AS Level Chemistry
- 6. Behaviour of Sub-atomic particles in an Electric Field Ѳ1 & Ѳ2 Angle of Deflection : More Factors: Charge of particle Mass of particle Electric field strength X ~ More ~ Less Ʇ- ve + ve P Ѳ2 is greater than Ѳ1 Since electron is lighter than proton Ʇ Ѳ2 Ѳ1 no p 1+ e 1- Atomic Structure Lecture- 3 AS Level Chemistry
- 7. ~ Relative Angle of Deflection ( RAD ) RAD α Charge RAD α 1 𝑀𝑎𝑠𝑠 RAD = 𝐶ℎ𝑎𝑟𝑔𝑒 𝑀𝑎𝑠𝑠 X Given Ѳ a) 2H1 1+ b) 4He2 2+ c) 23Na11 1+ 19F9 1- a ) RAD for = X 4 = 2𝑜 d) d) RAD for 19F9 1- = − 1 19 X 4 = − 4 19 OR 4 19 but in opposite direction Ʇ Ʇ Ѳ = 4 o + ve - ve 1H1 1+ 2H1 1+ 1 2 Atomic Structure Lecture- 3 AS Level Chemistry
- 8. Extra-nuclear Part : ~ Electrons ~ Circular paths • Shells – Quantum Shells / Energy Levels / Energy Bands Definite amount of Energy – Chemical Energy of e1- = K.E. + P.E. Motion Distance Shell Name K L M N O P Q Number 1 2 3 4 5 6 7 Shell Number = Principal Quantum Number = n n= 1,2,3 ……. Ꝏ Value of n : o Size of shell o Energy of shell ~ Large ~More ~ More Number of Electrons in a Shell = 2 n2 Atomic Structure Lecture- 4 AS Level Chemistry
- 9. Within a Shell : ~ Electrons -- Small groups Subshells – / Sub-Energy Levels No. of Subshells = Shell No. } e 1- 2 = 2 x 12 18 8 32 = 2 x 22 = 2 x 32 = 2 x 42 Shell K = 1 L = 2 M = 3 N = 4 = Principal Quantum Number = n Atomic Structure Lecture- 4 AS Level Chemistry
- 10. Within a Sub-Shell : ~ Electrons -- Pair form ~ Pair of --- Orbital e 1- No . Of orbitals in a shell 2n2 2 OR n2 e1- ~ Tiny Magnet eA 1- eB 1- Shell K = 1 L = 2 M = 3 N = 4 Subshells 1 ~ 2 ~ 3 ~ 4 ~ s2 6 10 14 p s s s2 2 2 p p 6 6 d d 10 fX Subshell ~ s Orbitals 1 orbital Subshell ~ p Subshell ~ d 3 orbitals 5 orbitals Field : - Electric - Static - Magnetic - Moving } Atomic Structure Lecture- 4 AS Level Chemistry
- 11. Shapes Of Orbitals : ~ Shape / e1- Cloud / e1- Density ~ Sub-shell- p - 6 e1- -------- 3 orbitals - Equal E = Distance - but Different Orientation ( px , py , pz ) Degenerated Orbitals x y z x z y ~ Sub-shell- s Atomic Structure Lecture- 5 AS Level Chemistry Without orientation
- 12. s - Orbital Symmetrical Sphere Without orientation 2 – Lobed Dumb-bell p - Orbital d - Orbital 4 – Lobed Dumb-bell Atomic Structure Lecture- 5 AS Level Chemistry
- 13. Shapes Of s-Orbitals : Shapes Of p-Orbitals : 2s Atomic Structure Lecture- 5 AS Level Chemistry
- 14. Electronic Configuration : - THREE types o s p d f Notation o Noble Gas Core o Electrons-in-Boxes THREE Rules a) - Energy Rule b) - Degenerated orbital Rule c) - Orbital Rule Nucleus 2 2 3 3 3 4 4 s s p s p d s p 1 Energy increases E4s Is Less Than E3d - small size / More penetration 1s 2s 2p 3s 3p 3d 4s 4p OR 1s 2s 2p 3s 3p 4s 3d 4p 1s 2s 2p 3s 3p 3d 4s 4p Atomic Structure Lecture- 6 AS Level Chemistry
- 15. 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s 5p 5d 5f 6s 6p 6d 6f 7s 7p 4s 3d 4s is filled earlier than 3d Atomic Structure Lecture- 6 AS Level Chemistry
- 16. 20Ca = 1s2 2s2 2p6 3s2 3p6 4s2 2 8 8 2 13Al = 1s2 2s2 2p6 3s2 3p1 9F = 1s2 2s2 2p5 19K = 1s2 2s2 2p6 3s2 3p6 4s1 Noble Gas Core 2He 10Ne 18Ar 36Kr 54Xe 86Rn 20Ca = [ 18Ar ] n s2 4s2 / [ 18Ar ] 13Al = [ 10 Ne ] 3 s2 p1 3 9F = [ 2 He ] s2 2 2 p5 ~ Practice Work : - First 20 Elements ~ At.No. 1 to 20 Atomic Structure Lecture- 6 AS Level Chemistry
- 17. Electrons –in – Boxes : - Orbital OR OR ↓↑ ↓↑ 1H = 2He = 3Li = 4Be = 5B = 6C = 7N = 8O = 9F = 10Ne = 1s 1s 1s 1s 1s 1s 1s 1s 2s 2s 2s 2s 2s 2s 2p 2p 2p 2p Atomic Structure Lecture- 7 AS Level Chemistry ~ Completely Filled Subshell ~ Half - Filled Subshell ~ Partially Filled Subshell Most Stable 1s 2s 2p 1s 2s 2p Stable Least Stable
- 18. Electronic Configuration : ~ Transition Elements - Central Part of the Periodic Table ~ 1st Series - Atomic Number 21 to 30 21Sc 22Ti 23V 24Cr 25Mn 26Fe 27Co 28Ni 29Cu 30Zn [ 18Ar ] x = 2 ( Except ‘Cr’ & ‘Cu’ ; x = 1 ) 4sx 3dy y = 1 - 10 } 21Sc = 24Cr = 29Cu = [ 18Ar ] [ 18Ar ] [ 18Ar ] 4s1 4s2 4s1 3d10; 3d5 ; 3d1 ; 4s 4s 4s 3d 3d 3d 4s2 4s2 3d4 3d9 X X X X Atomic Structure Lecture- 8 AS Level Chemistry
- 19. Electronic Configuration : ~ Ions + ve - ve 2 1 -------- Loss of e 1- -------- Gain of e 1- }~ Valence Shell 20Ca2+ = 1s2 2s2 2p6 3s2 3p6 26Fe2+ = 35Br1- = 26Fe = [ 18Ar ] [ 18Ar ] 4s2 4s 3d6 3d6 3d6 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 Atomic Structure Lecture- 8 AS Level Chemistry
- 20. Ionization Energy : Ionization - Oxidation 1 2 Reduction - ‘E’ involved - ‘E’ involved ----------- Ionization Energy ----------- Electron Affinity All the Materials can lose e1- All the e1- can be lost ~ Plasma X ( s ) X ( g ) Heat Sublimation X1+ ( g ) + Heat Ionization e1- X1+ ( g ) + e1- e1- e1- X1+ ( g ) X2+ ( g ) + X3+ ( g ) + X2+ ( g ) X0 ( g ) ΔΗie1 ΔΗie2 ΔΗie3 + Atomic Structure Lecture- 9 AS Level Chemistry
- 21. ( ΔΗie1) Na ( ΔΗie1) Na( g ) Na1+ ( g ) + H2O( g ) H2O1+ ( g ) + H2SO4 2+ ( g ) H2SO4 3+ ( g ) + ( ΔΗie3) H2O H2SO4 e1- e1- e1- ( ΔΗie2) Ca Ca1+ ( g ) Ca2+ ( g ) + e1- Ca1+ ( g ) Ca3+ ( g ) + M4+ ( g ) M7+ ( g ) + 2 e1- 3 e1- ~ Sum of 2nd , 3rd I.E. - Ca ~ Sum of 5th , 6th , 7th I.E. - M ~ Write An Equation for the 1st I.E. of Oxygen. ~ Write An Equation for the 1st I.E. of Oxygen Gas. O2(g) O2 1+ (g) + e1- O1+ (g) + e1- O(g) Atomic Structure Lecture- 9 AS Level Chemistry
- 22. Ionization Energy : - Definition : 1 X1+ ( g ) + 1 e1- 1 e1- 1 e1- 1 X1+ ( g ) 1 X2+ ( g ) + 1 X3+ ( g ) + 1 X2+ ( g ) 1 X0 ( g ) ΔΗie1 ΔΗie2 ΔΗie3 The Enthalpy Change When One mole of e1- is removed Ca1+ ( g ) Ca2+ ( g ) + e1- ~ KJ. mol -1 -Endothermic ΔΗie1 ΔΗie2 ΔΗie3 ˂ ˂ ………… Applications : - Reactivity - Valency - Distribution of e1- X O O O O O O O O O O O O N Atomic Structure Lecture- 10 AS Level Chemistry
- 23. Factors ( Ionization Energy ) : - Atomic Radius 1 2 3 - Nuclear Charge - Shielding / Screening - Atomic Radius 1 3Li = 2,1 11Na = 2,8,1 19K = 2,8,8,1 2 - Nuclear Charge Number of p1+ 3 - Shielding / Screening = Repulsion - Intervening shells / e1- - Reactive Metal = Low I . E. = Large Radius ; Low N.C. ; More Shielding Atomic Structure Lecture- 11 AS Level Chemistry 3Li 4Be 5B 6C 7N 8O 9F 10Ne
- 24. 3Li 4Be 5B 6C 7N 8O 9F 10Ne 1 2 3 4 5 6 7 8 Group : ~ Period # 2: Questions : 1. Trend of I.E. Across the Period 2,1 2,2 2,3 2,4 2,5 2,6 2,7 2,8 1+ 2+ 3+ 4+ 5+ 6+ 7+ 8+ o Number of Shells / Shielding remains same o Nuclear Charge increases o Nuclear Force / Pull increases o Atomic Radius decreases o I .E. increases o Nuclear Charge increases ( Ionization Energy ) Atomic Structure Lecture- 12 AS Level Chemistry
- 25. 3Li = 2,1 11Na = 2,8,1 19K = 2,8,8,1 Q.2. Trend of I.E. Down the Group o Number of Shells / Shielding increases o Increase in Nuclear Charge cancels by increased Shielding o Nuclear Force / Pull decreases o Atomic Radius increases o I .E. decreases o Number of Shells / Shielding increases Atomic Structure Lecture- 12 AS Level Chemistry
- 26. - Q.3. I.E. values Vs Order of e1- removed for the Element Sodium , Na : 11Na = 1s2 2s2 2p6 3s1 e1- Number Subshell 1 2-7 8-9 10-11 3s 2p 2s 1s Three Changes : 1 ~ Sudden Change 2 ~ Slight Change 3 ~ Steady Change I.E. Values / KJ.mol-1 1 2 3 4 5 6 7 8 9 10 11 e1- Removed Number X X X X X X X X X X X ---------- Change of Shell ---------- Change of Sub-Shell ---------- Change of Orbitals within a Sub-Shell - No. of e1- removed before 1st sudden Change = Valence e1- = Group Number }1 3 }2 } 1 3 3 Atomic Structure Lecture- 13 AS Level Chemistry
- 27. Q.4.The For Element X------ K.J .mol -1 First few I.E. Values 578 , 1930 , 2780 , 9200 , 11280 , 14500 i) Group # 3 ii) Formula of Oxide X O O O X3 O2 = X2O3 / Chloride XCl3 iii) s2 p1 n n Q.5. ~ Element M ------------ K.J. mole-1 430 , 920 , 1830 , 2830 , 9660 , 11220 Q.6. 1060 , 1907 , 2920 , 4960 , 6275 , 21265 , 25431 ~ Element X ------------ K.J. mole-1 A XCl B XCl2 C XCl3 D XCl4 Atomic Structure Lecture- 13 AS Level Chemistry
- 28. Q.7.Sketch a Graph for 1st I.E. Values Vs Atomic Number for the elements of Period # 2. ~ Period # 2: ~ Period # 3: 3Li 4Be 5B 6C 7N 8O 9F 10Ne 11Na 12Mg 13Al 14Si 15P 16S 17Cl 18Ar 3 4 5 6 7 8 9 10 Atomic Number 1st I.E. Values KJ.mol-1 X X X X X X X X Li Be B C N O F Ne ( Period # 3 ) II III V VI ~ Regular repeating pattern of properties across a period Periodicity O R Log I.E. Values Atomic Structure Lecture- 14 AS Level Chemistry 4 Be = 1s2 2s2 5 B = 1s2 2s2 2p1 7 N = 1s2 2s2 2p3 8 O = 1s2 2s2 2p4
- 29. Atomic Number 1st I.E. Values KJ.mol-1 X X X X X X X 2 4 5 6 7X 8 3 1 X X X X X X X X 1 2 3 4 5 6 7 8 Atomic Number I.E. Values X X X X A B C D ~ Sudden Change = Change Of Shell = Change Of Period Q.7. Give Reason , why ( 1st I.E. )Na is LESS than ( 1st I.E. )Mg But ( 2nd I.E. )Na is MORE than ( 2nd I.E. )Mg? 11Na 12Mg = 1s2 2s2 2p6 3 s1 = 1s2 2s2 2p6 3 s2 Atomic Structure Lecture- 14 AS Level Chemistry