Solid gas system
•Download as PPTX, PDF•
0 likes•461 views
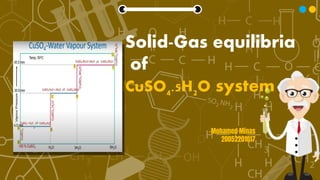
This document describes the solid-gas equilibria of the CuSO4-H2O system. It discusses how copper sulfate forms different hydrates - monohydrate, trihydrate, and pentahydrate - at increasing vapor pressures when water vapor is added at a constant temperature of 55°C. The phase diagram plots vapor pressure against water vapor composition, showing invariant equilibrium lines where two solid phases coexist with vapor, and monovariant lines where a single solid phase coexists with vapor. It also describes the dehydration of copper sulfate pentahydrate upon decreasing vapor pressure, passing through trihydrate and monohydrate before forming anhydrous copper sulfate.
Report
Share
Report
Share
Recommended
More Related Content
What's hot
What's hot (20)
Derivation of Bet equation and different isotherms

Derivation of Bet equation and different isotherms
Polynuclear Hydrocarbons Preparations and Reactions

Polynuclear Hydrocarbons Preparations and Reactions
Similar to Solid gas system
Similar to Solid gas system (20)
Phase diagram of a one component system ( water system )

Phase diagram of a one component system ( water system )
Recently uploaded
Model Call Girl Services in Delhi reach out to us at 🔝 9953056974🔝✔️✔️ Our agency presents a selection of young, charming call girls available for bookings at Oyo Hotels. Experience high-class escort services at pocket-friendly rates, with our female escorts exuding both beauty and a delightful personality, ready to meet your desires. Whether it's Housewives, College girls, Russian girls, Muslim girls, or any other preference, we offer a diverse range of options to cater to your tastes. We provide both in- call and out-call services for your convenience. Our in-call location in Delhi ensures cleanliness, hygiene, and 100% safety, while our out-call services offer doorstep delivery for added ease. We value your time and money, hence we kindly request pic collectors, time-passers, and bargain hunters to refrain from contacting us. Our services feature various packages at competitive rates: One shot: ₹2000/in-call, ₹5000/out-call Two shots with one girl: ₹3500 /in-call, ₱6000/out-call Body to body massage with sex: ₱3000/in-call Full night for one person: ₱7000/in-call, ₱10000/out-call Full night for more than 1 person : Contact us at 🔝 9953056974🔝. for details Operating 24/7, we serve various locations in Delhi, including Green Park, Lajpat Nagar, Saket, and Hauz Khas near metro stations. For premium call girl services in Delhi 🔝 9953056974🔝. Thank you for considering us Call Girls in South Ex (delhi) call me [🔝9953056974🔝] escort service 24X7![Call Girls in South Ex (delhi) call me [🔝9953056974🔝] escort service 24X7](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![Call Girls in South Ex (delhi) call me [🔝9953056974🔝] escort service 24X7](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
Call Girls in South Ex (delhi) call me [🔝9953056974🔝] escort service 24X79953056974 Low Rate Call Girls In Saket, Delhi NCR
Recently uploaded (20)
scipt v1.pptxcxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx...

scipt v1.pptxcxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx...
"Lesotho Leaps Forward: A Chronicle of Transformative Developments"

"Lesotho Leaps Forward: A Chronicle of Transformative Developments"
Block diagram reduction techniques in control systems.ppt

Block diagram reduction techniques in control systems.ppt
S1S2 B.Arch MGU - HOA1&2 Module 3 -Temple Architecture of Kerala.pptx

S1S2 B.Arch MGU - HOA1&2 Module 3 -Temple Architecture of Kerala.pptx
DC MACHINE-Motoring and generation, Armature circuit equation

DC MACHINE-Motoring and generation, Armature circuit equation
Hazard Identification (HAZID) vs. Hazard and Operability (HAZOP): A Comparati...

Hazard Identification (HAZID) vs. Hazard and Operability (HAZOP): A Comparati...
Bhubaneswar🌹Call Girls Bhubaneswar ❤Komal 9777949614 💟 Full Trusted CALL GIRL...

Bhubaneswar🌹Call Girls Bhubaneswar ❤Komal 9777949614 💟 Full Trusted CALL GIRL...
HOA1&2 - Module 3 - PREHISTORCI ARCHITECTURE OF KERALA.pptx

HOA1&2 - Module 3 - PREHISTORCI ARCHITECTURE OF KERALA.pptx
Standard vs Custom Battery Packs - Decoding the Power Play

Standard vs Custom Battery Packs - Decoding the Power Play
Call Girls in South Ex (delhi) call me [🔝9953056974🔝] escort service 24X7![Call Girls in South Ex (delhi) call me [🔝9953056974🔝] escort service 24X7](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![Call Girls in South Ex (delhi) call me [🔝9953056974🔝] escort service 24X7](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
Call Girls in South Ex (delhi) call me [🔝9953056974🔝] escort service 24X7
Solid gas system
- 1. Solid-Gas equilibria of CuSO4.5H2O system -Mohamed Minas 20052201017
- 2. There are such system in which two component forms a compound and at a particular temperature it decomposes into another solid and liquid. The composition of liquid state is different here then the solid state. At this point; Compd with Incg MP Original solid + solution These compounds are called as compound with Incongruent melting point. Actually the compound dissociate at this point and the process is called as peritectic reaction. The Incongruent melting point is called as meritectic or peritectic temperature. Incongruent melting point
- 3. Copper sulphate-water vapour system This is a two component system in which one component is solid and other component is water vapour. This phase diagram is drawn between vapour pressure v/s water vapour composition in such a way that by the addition of water vapour to the system at constant temp of 55°C copper sulphate forms three different hydrates at a certain vapour pressure level. The three hydrates are Copper sulphate monohydrate (CuSO4.H2O) Copper sulphate trihydrate(CuSO4.3H20) and Copper sulphate pentahydrate(CuSO4.5H20)
- 5. From Point O to A only Copper sulphate is present so there is an increase in vapour pressure of the system. Point A to B: Now monohydrate starts forming and system remains in equilibrium ; CuSO4 + H20 CuSO4.H20 at 4.5 mm of Hg. From B to C only monohydrate is present so again there is an increase in vapour pressure of the system. Point C to D: Now trihydrate starts forming and system remains in equilibrium ; CuSO4.H20 + 2H20 CuSO4.3H20 at 30.9 mm Hg. From D to E only trihydrate is present so again there is an increase in vapour pressure of the system. Point E to F: Now pentahydrate starts forming and system remains in equilibrium ; CuSO4.3H20 + 2H20 CuSO4.5H20 at 45.5 mm Hg.
- 6. From F to G only pentahydrate is present so only increase in vapour pressure of the system. At point O anhydrous CuSO4, has very low vapour pressure hence no reaction between CuSO4 and water, so by the addition of water vapour the vapour pressure of the system starts increasing. On line OA,BC,DE and FG two phases are present. One is either copper sulphate or its hydrate and other is water vapour so on applying phase rule; F’=C-P+1 ; F=2-2+1=2 i.e. system is monovariant at these lines. On line AB,CD and EF three phases are present. Two solids and one water vapour. There is an equilibrium between these three phases, on applying phase rule; ; F’=C-P+1 ; F=2-3+1=0 i.e. system is invariant at these lines.
- 7. Dehydration of Copper sulphate pentahydrate
- 8. Here the temperature is kept constant and the pressure over the salt is continuously decreased, till the dehydration of the salts and the dissociation equilibrium is set up. From Point A to B only Copper sulphate pentahydrate is present so there is an decrease in vapour pressure of the system. Point A to B: Now pentahydrate starts decomposing and system remains in equilibrium. From B to C only trihydrate is present so again there is an decrease in vapour pressure of the system. Point C to D: Now monohydrate starts forming and system remains in equilibrium. From D to E only monohydrate is present so again there is an decrease in vapour pressure of the system. Point E to F: Now anhydrous CuSO4 starts forming and system remains in equilibrium. The degree of freedom of the curve AB,CD,EF is zero i.e, invarient and degree of freedom of the curve B,D,F is 1 i.e, monovarient.