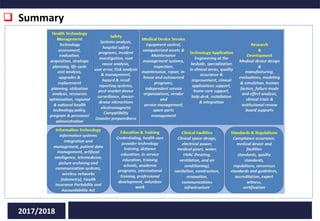
Clinical engineering represents the applications of biomedical engineering theories and methodologies to improve healthcare quality. Clinical engineers manage medical equipment in hospitals throughout its lifecycle, including selection, procurement, training, maintenance, and disposal. A full clinical engineering implementation involves diagnostic studies, inventory management, standards compliance, cost control, maintenance, training, and ensuring patient safety.
![Regents Biology 2015/2016
[ Clinical
Engineering ]
2017/2018
Supervised by :- Dr. Dalia H. Elkamchouchi
Allied Medical Science
Medical equipment technology](https://image.slidesharecdn.com/clinicalengineering-180106142338/85/Clinical-engineering-2-320.jpg)