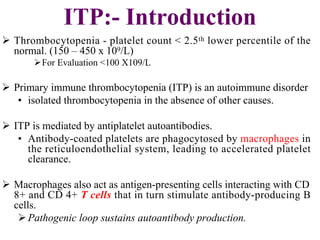
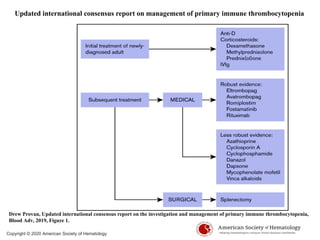
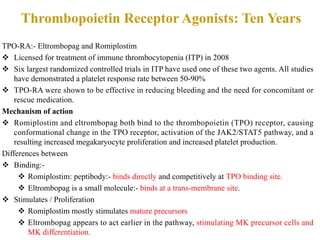
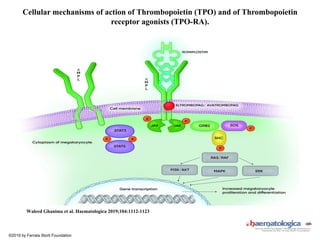
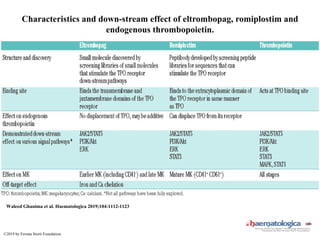
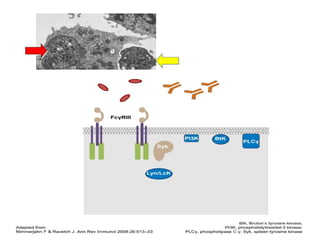
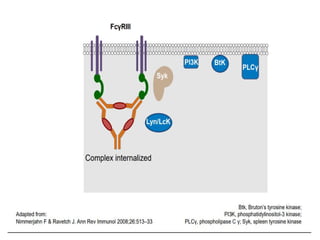
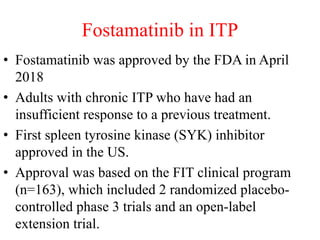
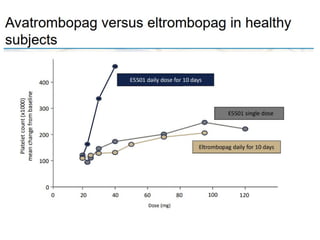
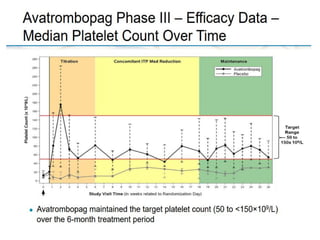
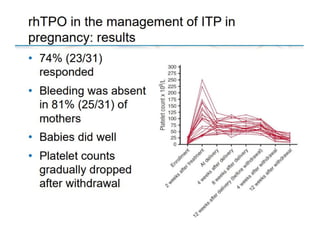
- Primary immune thrombocytopenia (ITP) is an autoimmune disorder characterized by isolated thrombocytopenia caused by autoantibodies against platelets.
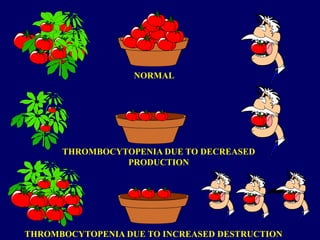
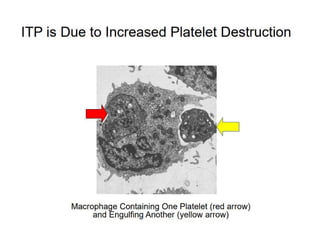
- ITP is mediated by antiplatelet autoantibodies which coat platelets, leading to their phagocytosis by macrophages and accelerated platelet clearance.
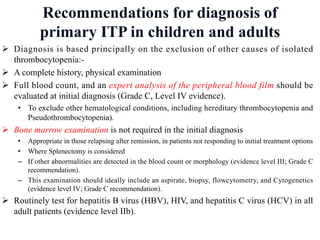
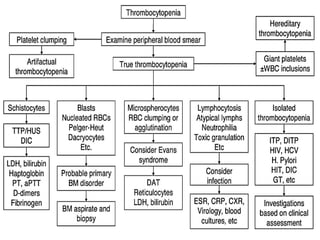
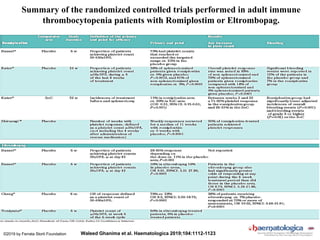
- Diagnosis of ITP involves excluding other causes of thrombocytopenia based on a complete history, physical exam, blood tests and blood smear. Bone marrow testing is usually not required for initial diagnosis.