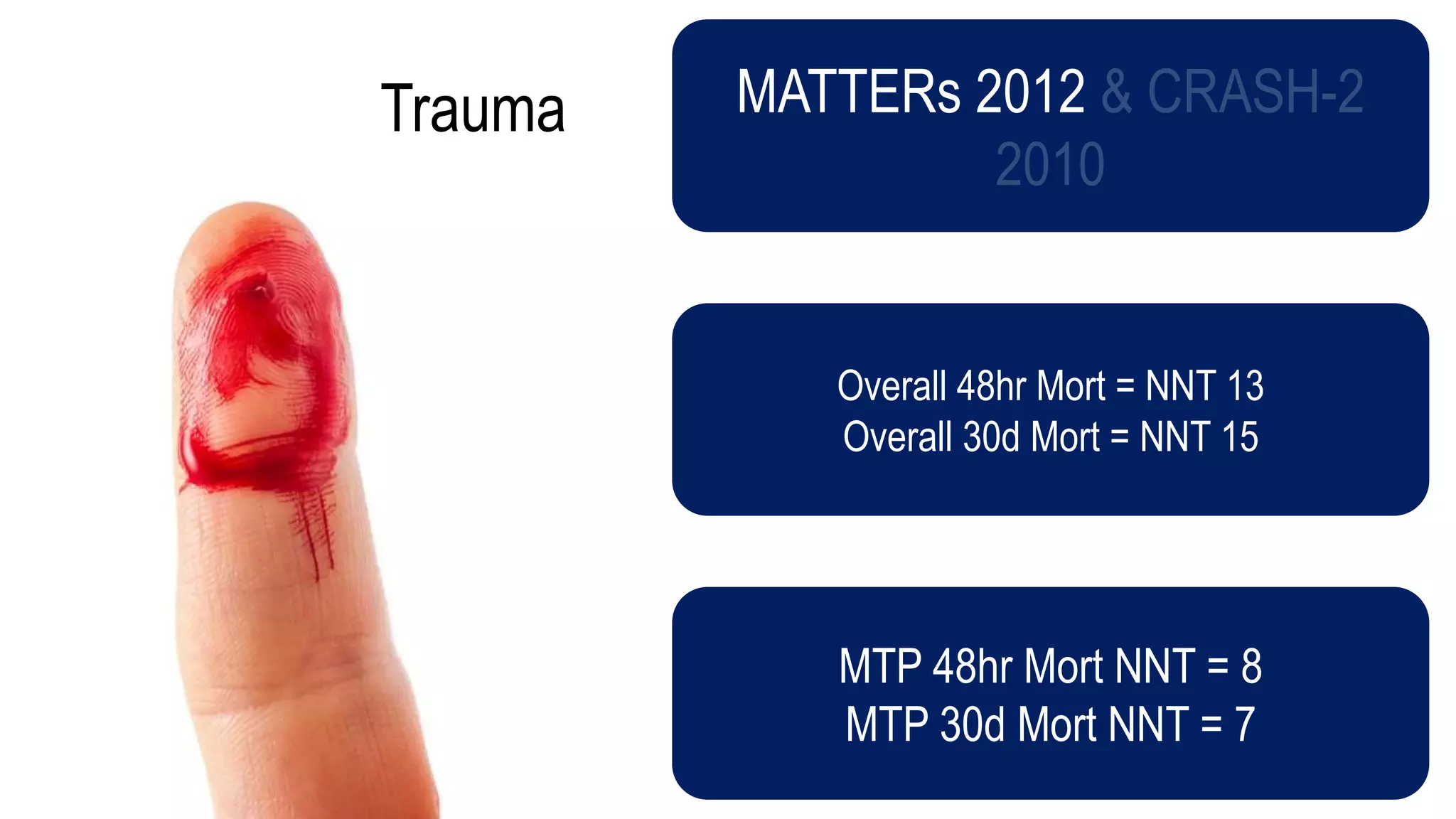
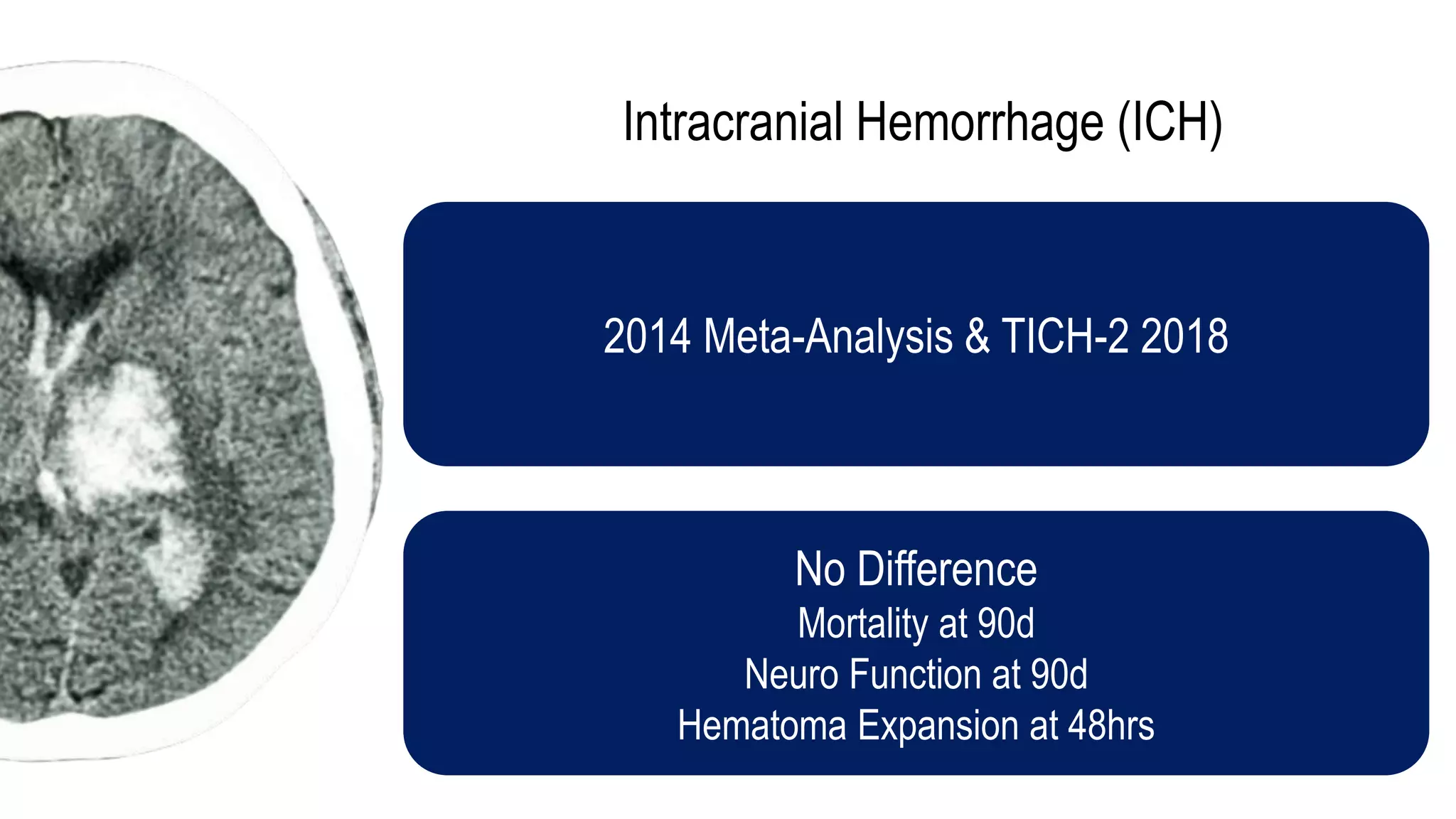
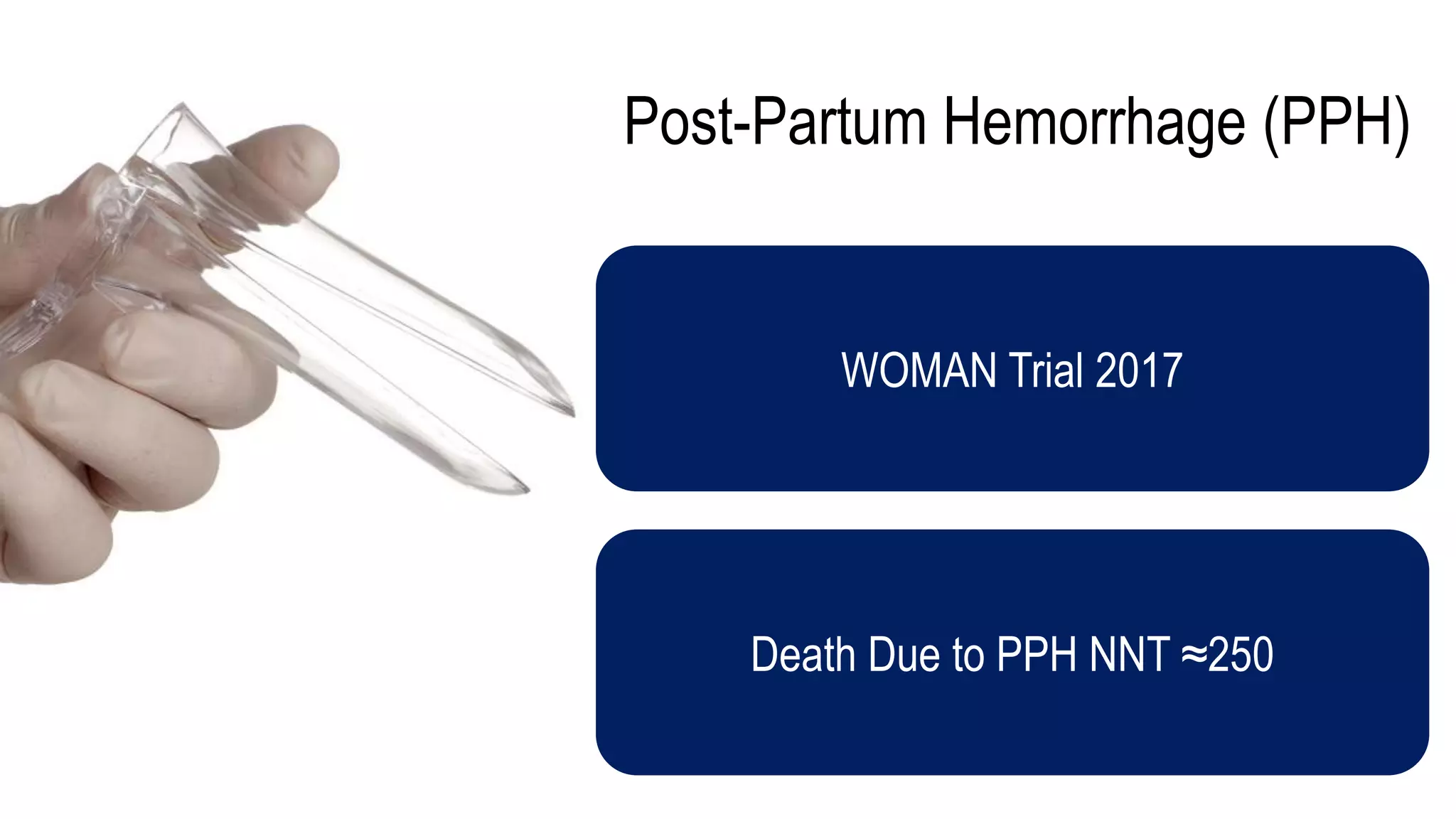
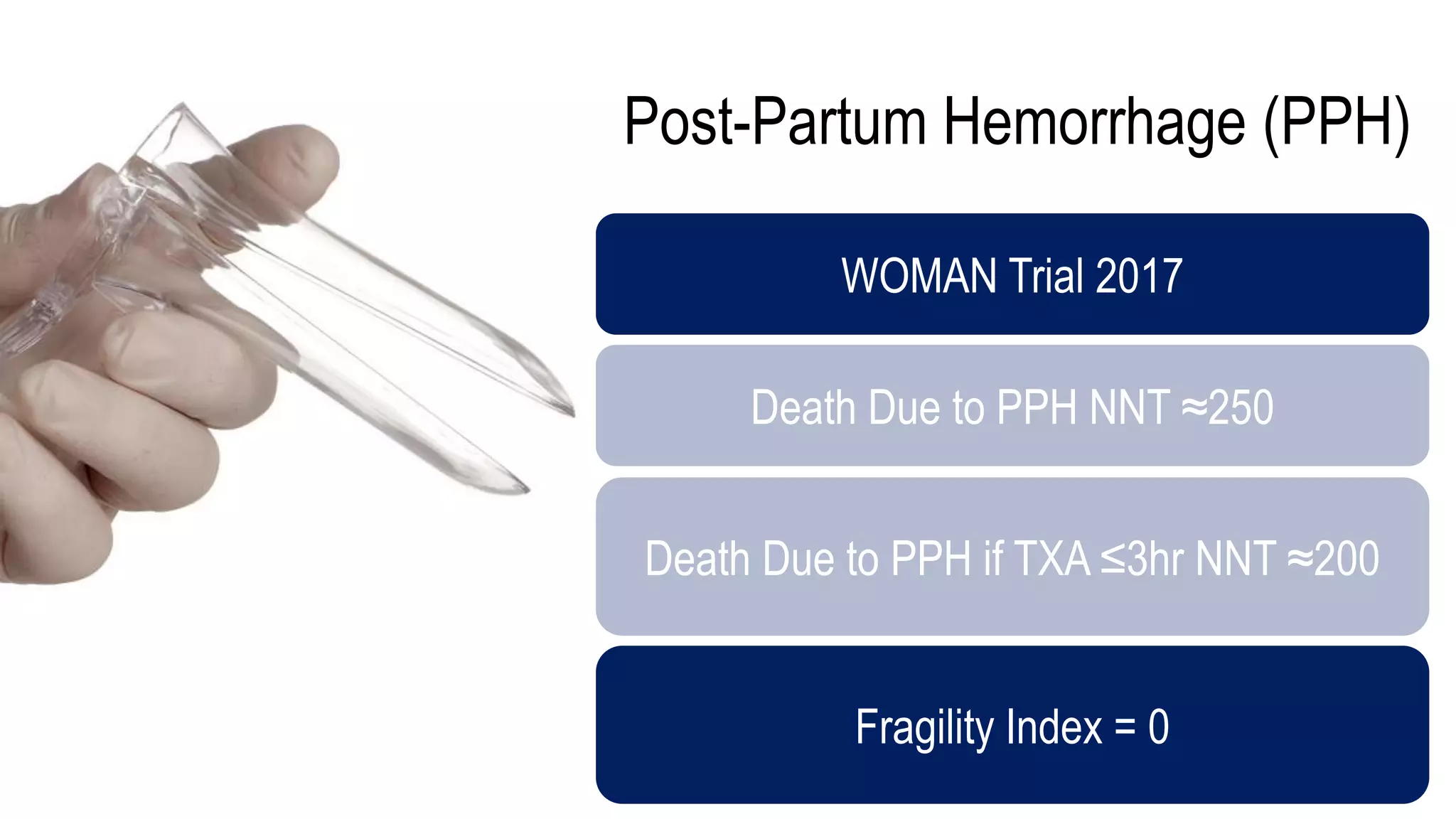
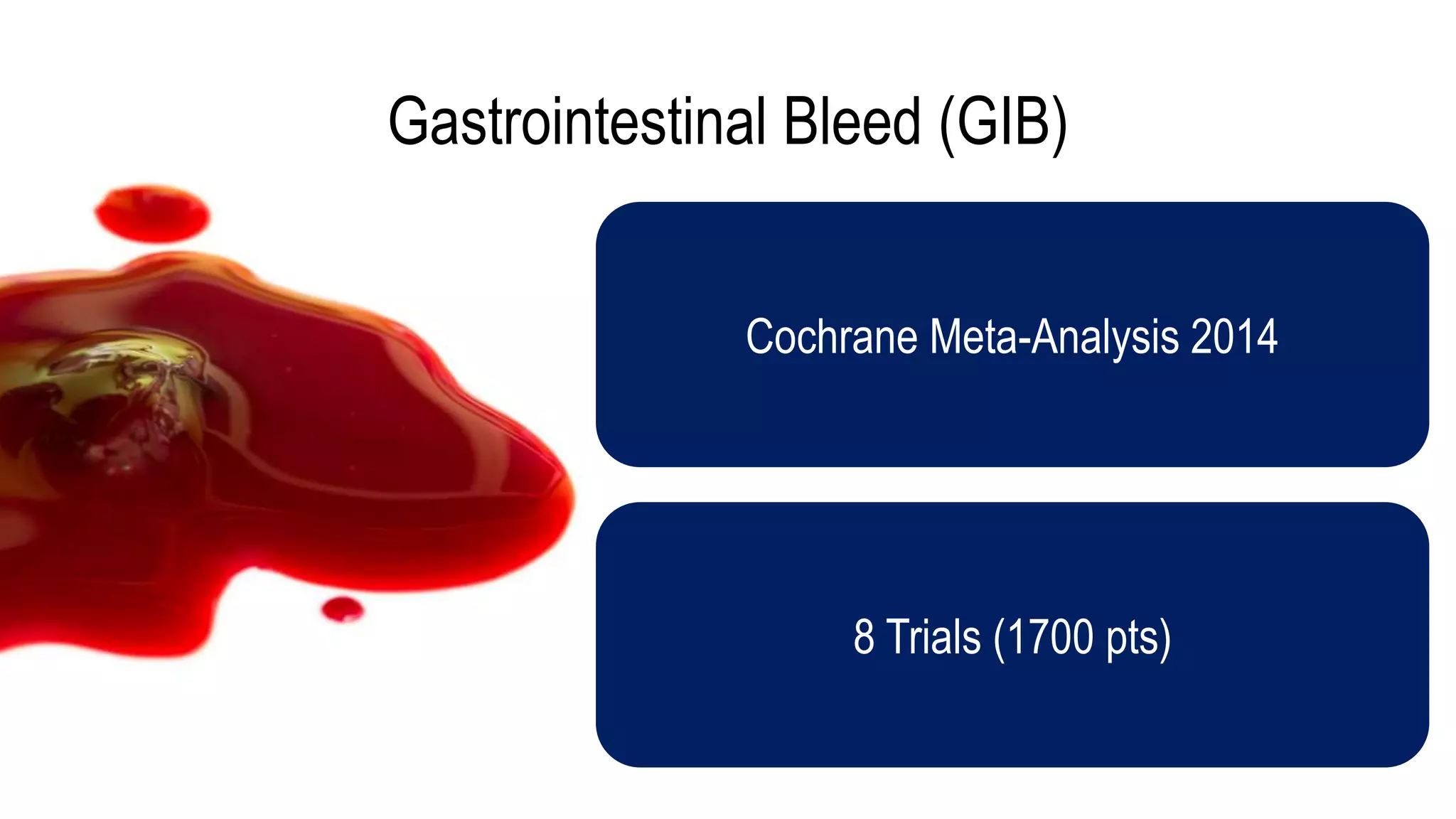
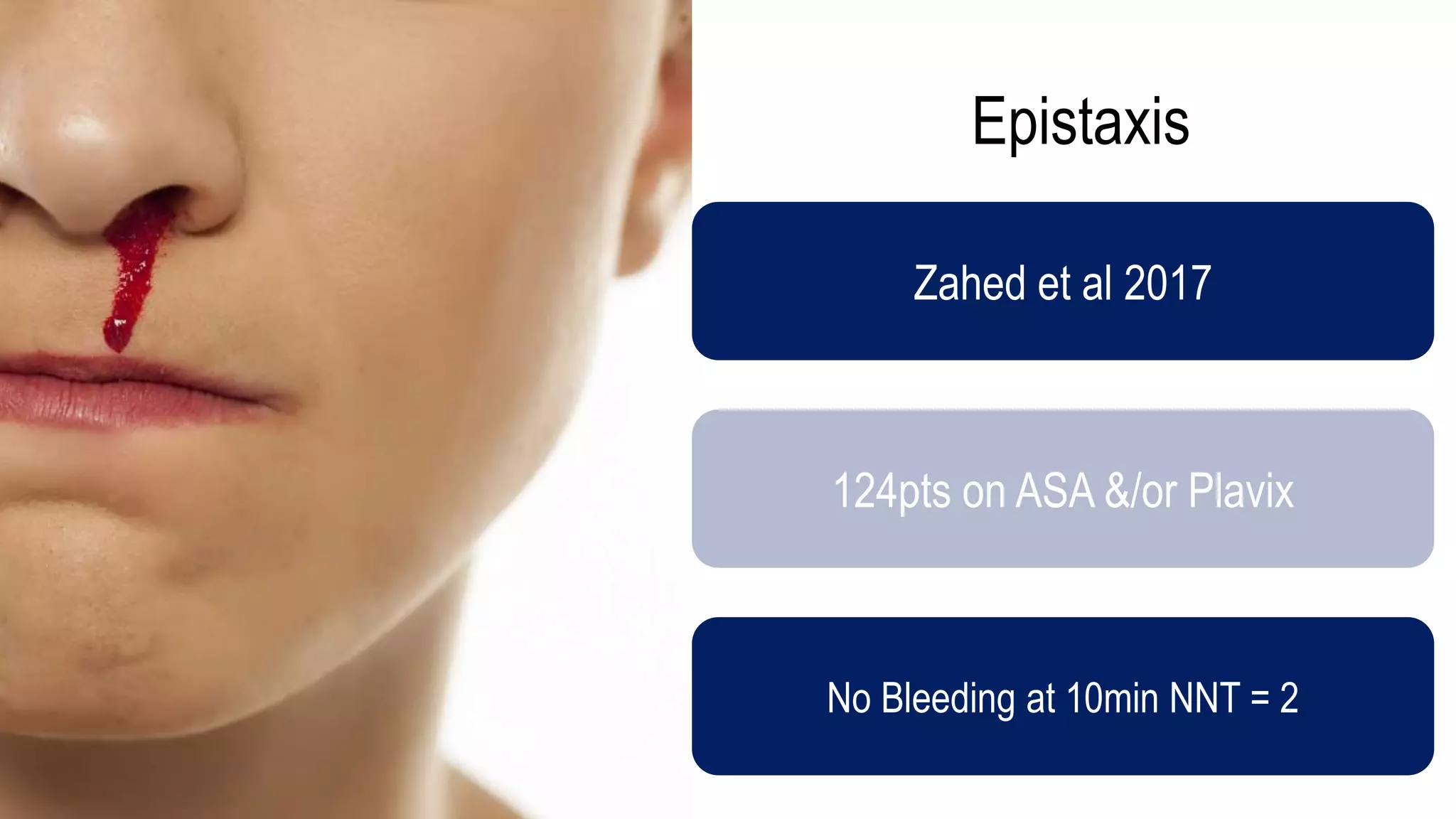
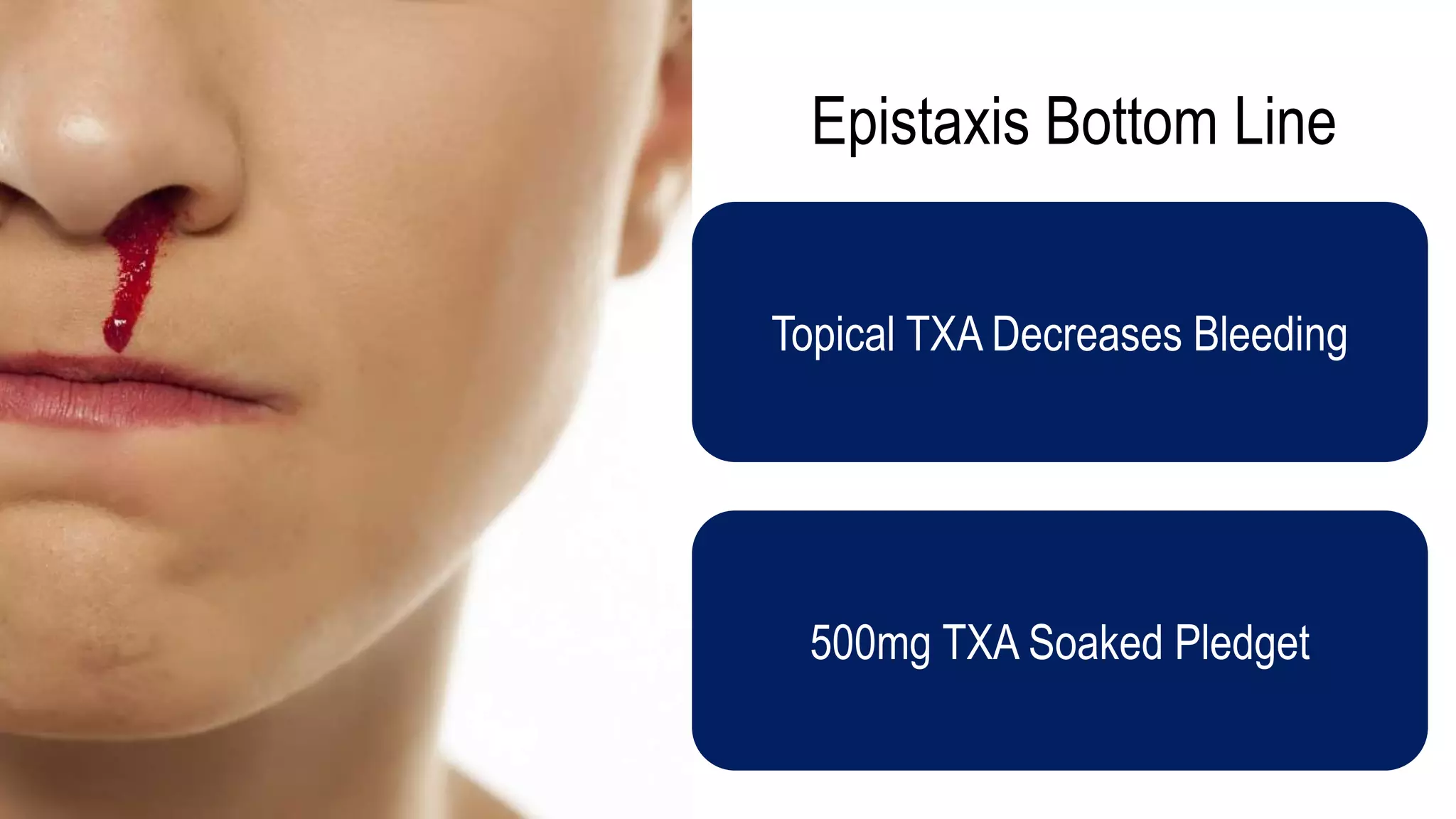
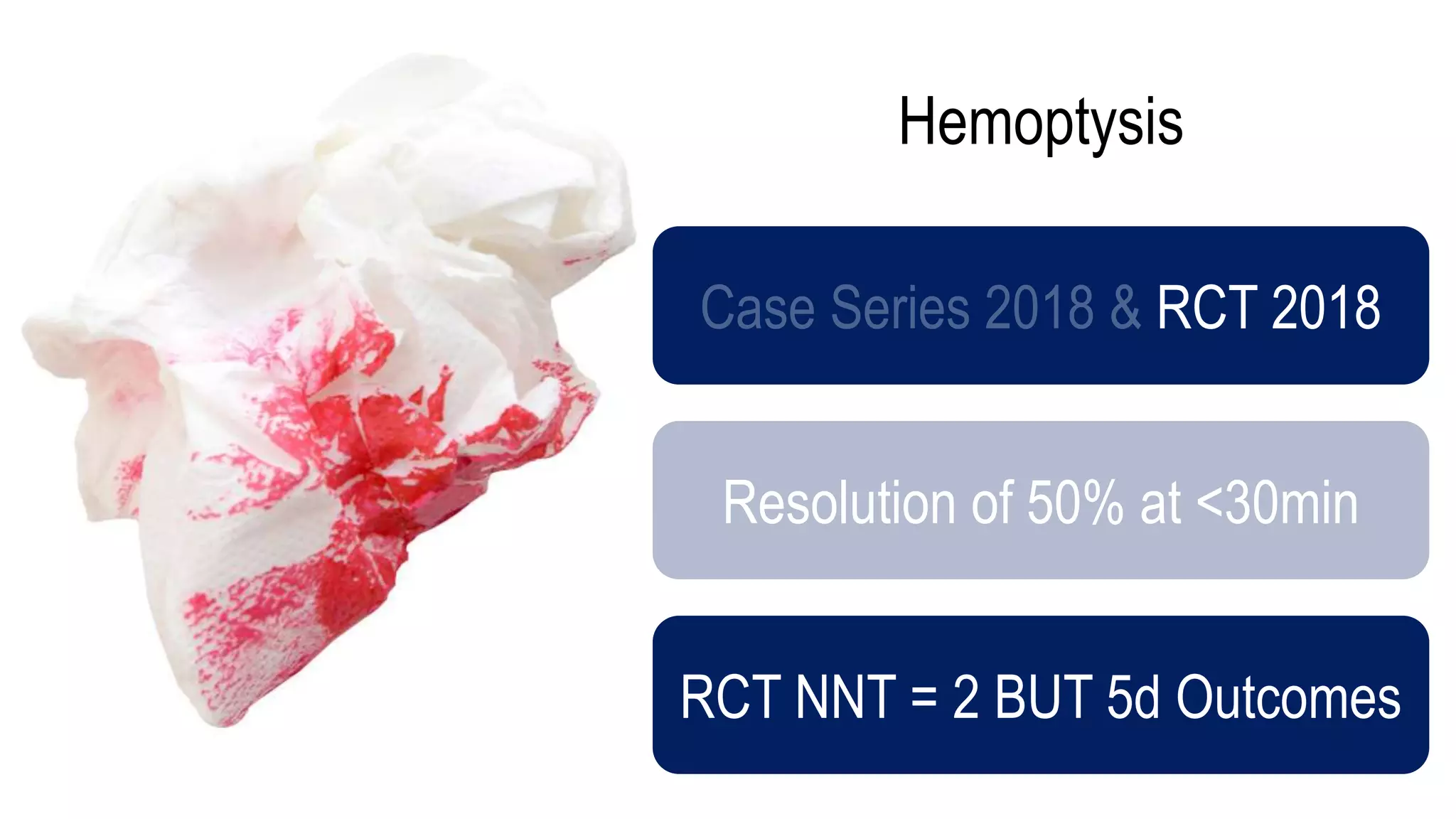
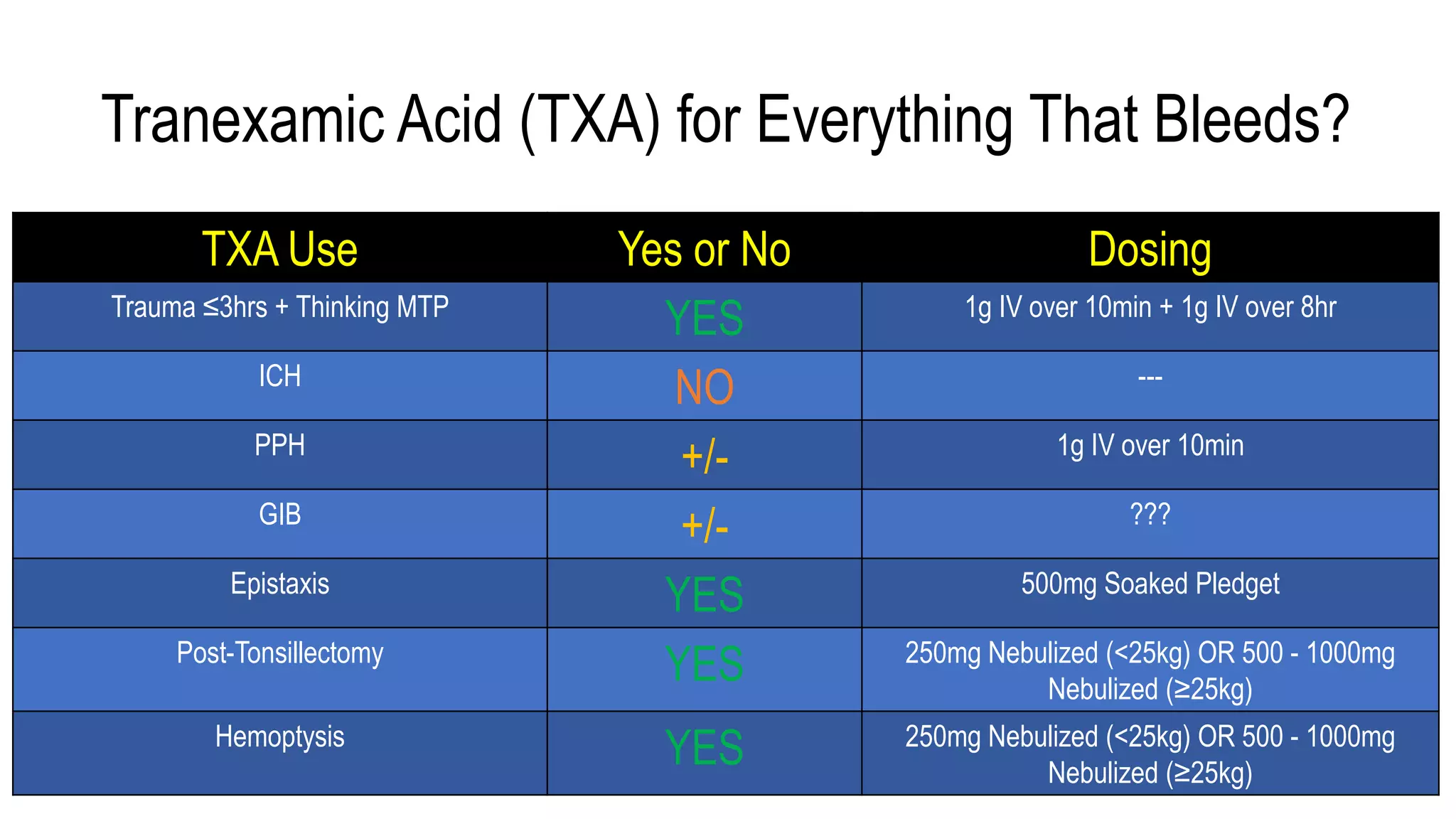
The document evaluates the efficacy of tranexamic acid (TXA) for various bleeding conditions, providing insights from studies such as the Crash-2 trial and several meta-analyses. Key findings suggest TXA is beneficial for trauma and gastrointestinal bleeding, while its use in intracranial hemorrhage and postpartum hemorrhage shows minimal patient-oriented benefits. The document concludes with an outline of recommended dosing and indicates scenarios where TXA usage is applicable.