Chapter14 140331231410-phpapp02
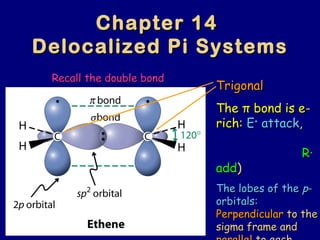
- 1. Chapter 14Chapter 14 Delocalized Pi SystemsDelocalized Pi Systems TrigonalTrigonal TheThe ππ bond is ebond is e-- rich:rich: EE++ attackattack,, R∙R∙ addadd)) The lobes of theThe lobes of the pp-- orbitals:orbitals: PerpendicularPerpendicular to theto the sigma frame andsigma frame and Recall the double bondRecall the double bond
- 2. 2-Propenyl (Allyl)2-Propenyl (Allyl) Question:Question: What about adding aWhat about adding a thirdthird pp-orbital adjacent to the double bond?-orbital adjacent to the double bond? Is there something special?Is there something special? Or: Is there any specialOr: Is there any special reactivity at the carbonsreactivity at the carbons adjacent to a double bond?adjacent to a double bond? H
- 3. a.a. b.b. c.c. SSNN1 reactivity of allylic carbon like that1 reactivity of allylic carbon like that of Rof RsecsecX,X, even though it is primary!even though it is primary! ppKKaa ~ 40:~ 40: Acidic!Acidic! 5050 SSNN11 87 kcal mol87 kcal mol-1-1 :: Weak!Weak! 101 kcal mol101 kcal mol-1-1 Replacing one of the hydrogens in ethene withReplacing one of the hydrogens in ethene with anotheranother spsp22 -hybridized carbon gives an-hybridized carbon gives an allylicallylic system.system. Allylic positionAllylic position Observations:Observations: H L H B B H H
- 4. Clearly: AllylicClearly: Allylic ·· areare stabilized.stabilized.-- ++ Short notation: Dotted linesShort notation: Dotted lines Why?Why? Resonance!Resonance! CH2 H2C CH2 H2C CH2 H2C
- 5. MO Picture of 2-PropenylMO Picture of 2-Propenyl (Allyl)(Allyl) 33 ppOs 3 MOsOs 3 MOs RecallRecall: Bonds made by overlap: Bonds made by overlap HH .. HH .. H HH H++ ++HH ++ ++HH ++HH++HH In phaseIn phase ++HH ++ --HH --HH++HH Out ofOut of phasephase BondingBonding Anti-Anti- bondingbonding NodeNode Sign of the wave function,Sign of the wave function, Not charge!Not charge! EE
- 6. EE ππ σσ σσ** ππ** CH2 CH2 What happens to this pictureWhat happens to this picture when we interact with anotherwhen we interact with another pp orbital?orbital?
- 7. 1)1) Interaction with theInteraction with the ππ bonding orbital causesbonding orbital causes energy splitting: theenergy splitting: the pp orbital level moves up andorbital level moves up and thethe ππ bonding level movesbonding level moves down.down. 2)2) Interaction with theInteraction with the ππ antibonding orbital causesantibonding orbital causes the energy level of thethe energy level of the pp orbital to move down toorbital to move down to where it was originally andwhere it was originally and the energy level of thethe energy level of the ππ antibonding orbital to moveantibonding orbital to move up. The two effects on theup. The two effects on the pp orbital cancel each otherorbital cancel each other out and the twoout and the two ππ orbitalsorbitals are pushed apart.are pushed apart. Interactions of a singly occupiedInteractions of a singly occupied pp-- orbital with each of theorbital with each of the ππ molecularmolecular orbitalsorbitals pp EE ππ ππ** 00 ππ ππ** upup downdown unchangedunchanged EtheneEthene AllylAllyl pp OrbitalOrbital NonbondingNonbonding MOMO
- 8. H2C H C CH2 pp EE # of e# of e dependsdepends onon ++,,∙∙,,-- ππ ππ** 00 Resulting picture:Resulting picture: AndAnd locationlocation atat terminitermini
- 9. Reactivity of AllylicReactivity of Allylic PositionPositionA. Radical HalogenationA. Radical Halogenation CHCH22 CHCHCHCH33 ++ BrBr22 CHCH22 CH CHCH CH22BrBr ++ HBrHBr Faster than addition!Faster than addition!Low conc.Low conc. Mech.Mech. 1.1. 2.2. BrBr22 2 Br2 Br ..hhυυ oror ΔΔ Initiation:Initiation: Propagation:Propagation: CHCH22 CHCHCHCH33 ++ BrBr .. CHCH22 CH CHCH CH22 .. CHCH22 CH CHCH CH22 .. ++ HBrHBr CHCH22 CH CHCH CH22 .. CHCH22 CH CHCH CH22 .. BrBr22 CHCH22 CH CHCH CH22Br +Br + BrBr ..
- 10. Termination:Termination: 3.3. BrBr .. ++ ..CHCH22CH CHCH CH22 BrCHBrCH22CH CHCH CH22 BrBr .. BrBr ..++ BrBr22 CHCH22CH CHCH CH22 ..22 CHCH22CH CHCH CH22CHCH22 CHCHCHCH22 Anything that traps radicals, including the “dirt”Anything that traps radicals, including the “dirt” on the walls of the flask, contributes toon the walls of the flask, contributes to termination.termination.
- 12. Propene generates a symmetrical allylic radical,Propene generates a symmetrical allylic radical, but this is not always the case. Forbut this is not always the case. For unsymmetrical systems: mixtures. Ratiosunsymmetrical systems: mixtures. Ratios depend on % radical character on each carbondepend on % radical character on each carbon and TSs leading to products.and TSs leading to products. Br2 Br· +
- 13. B. SB. SNN1: The Allylic Cation is Stabilized1: The Allylic Cation is Stabilized CHCH33CH CHCHCH CHCH22ClCl HH22OO -- ClCl -- CHCH33CH CH CHCH CH CH22 ++ CHCH33CH CH CHCH CH CH22 ++ CHCH33CH CHCHCH CHCH22OHOH CHCH33CHCH CHCHCH CH22 OHOH fastfastslowslow ThermodynamicThermodynamic productproduct Kinetic productKinetic product ThermodynamicThermodynamic KineticKinetic CationCation EE
- 14. C. SC. SNN2: The Allylic TS is Stabilized2: The Allylic TS is Stabilized CHCH33CH CHCHCH CHCH22ClCl NaNaII CHCH33CH CHCH CH CC ClCl II .. ++ ‡‡ CHCH33CH CHCHCH CHCH22II ++ ClCl -- 100 times faster100 times faster thanthan Cl .... δδ-- δδ--
- 15. D. Allylic OrganometallicsD. Allylic Organometallics Li CH3 CH3 ++ RXRX Allylic Grignard reagents:Allylic Grignard reagents: Br MgMg++ X Neutral analogs of allylic anions:Neutral analogs of allylic anions: NMR shielded!NMR shielded! X = OR, SR, NRX = OR, SR, NR22 H2C C CH3 CH2 Li H2C C CH3 CH2R MgBr X
- 16. Conjugated DoubleConjugated Double BondsBonds What aboutWhat about Nomenclature: Cis/trans E/Z reviewNomenclature: Cis/trans E/Z review C C C CC C C C ??
- 17. Stability:Stability: Heats of hydrogenationHeats of hydrogenation CHCH33(CH(CH22))33CH CHCH CH22 HH22++ 1,5-Hexadiene1,5-Hexadiene 1,3-Butadiene1,3-Butadiene But:But: Resonance energy of butadiene ~ 3.5Resonance energy of butadiene ~ 3.5 ΔΔH˚H˚ -30.3-30.3 -60.5-60.5 -57.1-57.1 2 H2 H22++ 2 H2 H22++
- 18. StructureStructure Short! (1.54 Å normal)
- 19. OrbitalsOrbitals
- 20. NMRNMR H H H H JJ = 10= 10 δδ = 5.06= 5.06 δδ = 6.27= 6.27 (effect of 2(effect of 2ndnd double bond)double bond) JJ ~1-2~1-2 δδ = 5.16= 5.16 JJtranstrans = 17= 17 JJciscis = 10= 10 H H H H 137.2137.2 116.6116.6
- 21. ConjugationConjugation stabilizes thermodynamically, but it also increasesstabilizes thermodynamically, but it also increases reactivity, for example inreactivity, for example in electrophilic additionselectrophilic additions (review Chapter 12).(review Chapter 12). 1,2-Addition1,2-Addition (kinetic)(kinetic) Intermediate cation is also stabilizedIntermediate cation is also stabilized ++ HClHCl ClCl -- 1,4-Addition1,4-Addition (thermodynamic)(thermodynamic) CH3 ClCl CH3 ClCl CH3 CH3 ClCl -- + cis+ cis HClAddnHClAddn
- 22. Kinetic vs ThermodynamicKinetic vs Thermodynamic ControlControl
- 24. Extended ConjugationExtended Conjugation Thermo 1Thermo 1 Thermo 2Thermo 2 KineticKinetic ++ HHBrBr BrBr-- Three productsThree products CH3 CH3 CH3 Quite reactiveQuite reactive The more double bonds, the more sensitiveThe more double bonds, the more sensitive (reactive) is the polyene.(reactive) is the polyene.
- 25. Cyclohexatriene is Special -Cyclohexatriene is Special - BenzeneBenzene Cyclic arrayCyclic array ofof six electronssix electrons has special stability,has special stability, calledcalled aromaticityaromaticity (Chapter 15).(Chapter 15). Benzene is relatively inert to HBenzene is relatively inert to H22-cat, electrophiles,-cat, electrophiles, oxidants, in comparison with hexatriene.oxidants, in comparison with hexatriene.
- 26. Extended Conjugation in NaturalExtended Conjugation in Natural and Unnatural Productsand Unnatural Products Orange color of carrotsOrange color of carrots BiologicalBiological degradationdegradation VisionVision
- 27. Organic ConductorsOrganic Conductors Heeger, MacDiarmid, Shirakawa, Nobel Prize 2000
- 28. Light emitting diodes (LEDs)Light emitting diodes (LEDs)
- 29. Conjugated Systems UndergoConjugated Systems Undergo Special Transformations:Special Transformations: Pericyclic ReactionsPericyclic Reactions The conjugatedThe conjugated ππ system can react as a unit,system can react as a unit, involvinginvolving both endsboth ends. For example,. For example, 1. Cycloadditions:1. Cycloadditions: TheThe Diels-Alder reaction,Diels-Alder reaction, a [4+2] cycloadditiona [4+2] cycloaddition ++ ΔΔ 44ππ-4C-4C DieneDiene 22ππ-2C-2C DienophileDienophile 20%20% CycloadductCycloadduct HC HC CH2 CH2 H2 C C H2 HC CH2 HC CH2 CH2 CH2
- 30. Diels-Alder reactions work best when we pair anDiels-Alder reactions work best when we pair an e-riche-rich (push)(push) dienediene with anwith an e-poore-poor (pull)(pull) dienophiledienophile e-poor dienee-poor diene with anwith an e-rich dienophilee-rich dienophile or anor an The Diels-Alder Reaction isThe Diels-Alder Reaction is ChemoselectiveChemoselective Depends on substituents:Depends on substituents: e-Donating:e-Donating: Alkyl, alkoxy, alkylthioAlkyl, alkoxy, alkylthio CHCH33,, CHCH33O,O, CHCH33CHCH22SS HyperconjugationHyperconjugation ResonanceResonance Even though O is e-negative (inductive effect), resonance wins out.Even though O is e-negative (inductive effect), resonance wins out. OCH3 OCH3 OCH3
- 31. e-Withdrawing:e-Withdrawing: CFCF33, CR, C N, NO, CR, C N, NO22 OO Resonance:Resonance: Example:Example: Inductive:Inductive: Does not compete with dienophileDoes not compete with dienophile 90%90% ++ ΔΔ C F F F H2C C CR H O H2C C CR H O H2C C CR H O CR O CR O
- 32. ++ ΔΔ Orbital description:Orbital description: spsp33 Mechanism: ConcertedMechanism: Concerted spsp22 spsp22
- 33. ConsequencesConsequences Stereospecific: Retention ofStereospecific: Retention of Dienophile StereochemistryDienophile Stereochemistry ++ ++ CisCis CisCis TransTrans TransTrans 80%80% 90%90% COCH3 CH3 O COCH3 O H3C COCH3 O CH3 COCH3 O CH3
- 34. Retention of Diene StereochemistryRetention of Diene Stereochemistry OCH3 OCH3 NC CN CNNC OCH3 OCH3 ++ ++ NC CN CNNC When both partners are stereochemically defined:When both partners are stereochemically defined: “Endo rule” determines their approach.“Endo rule” determines their approach. CN CN CN CN OCH3 OCH3 CN CN CN CN OCH3 OCH3
- 35. Endo/Exo AdditionEndo/Exo Addition Substituents point away from diene Substituents point toward diene DADA
- 36. o o i i A A Generally:Generally: ++ ExampleExample:: EndoEndo o i io A A CH3 H3C NC H HNC CH3 H3C CN CN WalbaWalba LipshutzLipshutz DylanDylan SegoviaSegovia
- 37. Alkynes as DienophilesAlkynes as Dienophiles Generates 1,4-cyclohexadienesGenerates 1,4-cyclohexadienes CO2CH3 CO2CH3 CO2CH3 CO2CH3 ++ Can reactCan react againagain 75%75% CO2CH3 CO2CH3
- 38. ΔΔ ΔΔ hhυυ ExothermicExothermic (ring strain(ring strain released)released) Light driven:Light driven: Can beat thermodynamicsCan beat thermodynamics.. Wavelength dependent (can go either way).Wavelength dependent (can go either way).ΔΔH °H ° = -9.7 kcal mol= -9.7 kcal mol-1-1 ExothermicExothermic (C C better than C C, unless ring strain present)(C C better than C C, unless ring strain present) ΔΔH °H ° = -14.7 kcal mol= -14.7 kcal mol-1-1 hhυυ 2. Electrocyclic Reactions:2. Electrocyclic Reactions: Intramolecular ring closure and openingsIntramolecular ring closure and openings
- 39. Electrocyclic ReactionsElectrocyclic Reactions are Stereospecificare Stereospecific CH3 CH3 CH3 CH3 ΔΔ ciscis–3,4-Dimethylcyclobutene–3,4-Dimethylcyclobutene TransTrans Only!Only! Only!Only! cis,transcis,trans–2,4-Hexadiene–2,4-Hexadiene Trans,transTrans,trans ΔΔ
- 40. Movement ofMovement of SubstituentsSubstituents ConrotatoryConrotatory Conrotatory:Conrotatory: They rotate in theThey rotate in the same directionsame direction ΔΔ H CH3 H CH3 H CH3 CH3 H
- 41. ConrotatoryConrotatory (counterclockwise)(counterclockwise) Clockwise conrotation in principle possible butClockwise conrotation in principle possible but sterically prohibitedsterically prohibited:: ΔΔ ΔΔ CH3 H H CH3 CH3 H H3C H H CH3 H CH3 CH3 H H CH3
- 42. hhυυ disdis hhυυ disdis Fascinatingly, hFascinatingly, hυυ goesgoes disrotatorydisrotatory (rotation in(rotation in opposite directionsopposite directions)) CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3
- 43. ΔΔ = dis= dis Even more startling: The hexatriene/cyclohexadieneEven more startling: The hexatriene/cyclohexadiene interconversion is alsointerconversion is also stereospecific,stereospecific, but follows thebut follows the oppositeopposite rules of sense of rotation, compared torules of sense of rotation, compared to butadiene/cyclobutene system:butadiene/cyclobutene system:
- 44. hhυυ = con= con
- 45. Orbital Symmetry: An inkling of how this might go……Orbital Symmetry: An inkling of how this might go…… Conrotatory Disrotatory Controls thermalControls thermal closureclosure Controls photo-Controls photo- chemical closurechemical closure hhνν-provides e to-provides e to ππ33 The orbital signs at theThe orbital signs at the termini alternate with #termini alternate with # of double bonds.of double bonds.
- 46. ElectronicElectronic SpectroscopySpectroscopy (Ultraviolet-Visible or(Ultraviolet-Visible or UV)UV) White (sun) light is composedWhite (sun) light is composed of the visible spectrumof the visible spectrum RememberRemember spectroscopy:spectroscopy: EE Excited stateExcited state Ground stateGround state ΔΔEE == hhυυ == hhcc//λλ
- 47. UV-Vis spectroscopy requires much higher energy thanUV-Vis spectroscopy requires much higher energy than NMR (kcals vs calories), does not need external “condition”NMR (kcals vs calories), does not need external “condition” (magnet), built into molecule: electronic excitation from(magnet), built into molecule: electronic excitation from bonding to antibonding levels, particularly easy forbonding to antibonding levels, particularly easy for ππ systems, because occupiedsystems, because occupiedunoccupiedunoccupied ΔΔEE relatively small.relatively small. NoNo ππ bond left!bond left! Light causesLight causes cis-transcis-trans isomerization,isomerization, radicalradical reactionsreactions SimpleSimple ππ bond, as in ethene:bond, as in ethene:
- 48. Spectrum of EtheneSpectrum of Ethene Quoted asQuoted as λλmaxmax Broad, because of rotational andBroad, because of rotational and vibrational states. Electronicvibrational states. Electronic spectroscopy is fast, nospectroscopy is fast, no “averaging”“averaging”AA 171 nm171 nm WavelengthWavelength λλ (given in(given in nmnm, units of 10, units of 10-9-9 m; not in frequencym; not in frequency υυ = c/= c/λλ,, as we did in NMR, whereas we did in NMR, where λλ ~ 100 mm to 1m!)~ 100 mm to 1m!)
- 49. UV spectroscopy below 200 nm requires vacuum,UV spectroscopy below 200 nm requires vacuum, because air absorbs. Normally (in atmosphere) one scansbecause air absorbs. Normally (in atmosphere) one scans 200-400 (UV), 400-800 nm (visible). This allows lower200-400 (UV), 400-800 nm (visible). This allows lower energy tranisitons to be recorded, e.g. 1,3-butadiene:energy tranisitons to be recorded, e.g. 1,3-butadiene: εε == AA//cc EE RelativelyRelatively lowlow energyenergy Shoulder, sh Peak heights are reported asPeak heights are reported as εε :: Extinction coefficientExtinction coefficient,, which iswhich is absorbanceabsorbance normalized bynormalized by concentrationconcentration:: λmax λλmaxmax = 222.5 nm (εε == 10,800)
- 50. AbsorptionAbsorption in thein the visiblevisible 450 nm450 nm orangeorange--redred 550 nm550 nm violetviolet 650 nm650 nm blueblue--greengreen Color ofColor of substancesubstance Visible Absorption:Visible Absorption: ColorColor Light enters the prism from the top right, and is refracted by the glass. The violet is bent more than the yellow and red, so the colors separate. NewtonNewton
- 51. In extendedIn extended ππ systems many transitions aresystems many transitions are possible, giving rise to more complex and notpossible, giving rise to more complex and not readily interpretable spectra, but HOMO-LUMOreadily interpretable spectra, but HOMO-LUMO gap gets smaller: Longest wavelength absorptiongap gets smaller: Longest wavelength absorption is indicative of theis indicative of the extentextent of conjugation, e.g.of conjugation, e.g. CH3 λλ max = 271 nmmax = 271 nm ConjugatedConjugated λλ max = 217 nmmax = 217 nm UnconjugatedUnconjugated Greater conjugation:Greater conjugation: Smaller HOMO/LUMO gapSmaller HOMO/LUMO gap
Editor's Notes
- Walba 2:41 Lipshutz 2:28