Thomson atom model
•Download as PPTX, PDF•
1 like•737 views
student made
Report
Share
Report
Share
Recommended
More Related Content
What's hot
What's hot (20)
Properties and Formation of Ionic Compounds Powerpoint

Properties and Formation of Ionic Compounds Powerpoint
Similar to Thomson atom model
Similar to Thomson atom model (20)
Recently uploaded
https://app.box.com/s/x7vf0j7xaxl2hlczxm3ny497y4yto33i80 ĐỀ THI THỬ TUYỂN SINH TIẾNG ANH VÀO 10 SỞ GD – ĐT THÀNH PHỐ HỒ CHÍ MINH NĂ...

80 ĐỀ THI THỬ TUYỂN SINH TIẾNG ANH VÀO 10 SỞ GD – ĐT THÀNH PHỐ HỒ CHÍ MINH NĂ...Nguyen Thanh Tu Collection
Recently uploaded (20)
Interdisciplinary_Insights_Data_Collection_Methods.pptx

Interdisciplinary_Insights_Data_Collection_Methods.pptx
NO1 Top Black Magic Specialist In Lahore Black magic In Pakistan Kala Ilam Ex...

NO1 Top Black Magic Specialist In Lahore Black magic In Pakistan Kala Ilam Ex...
Python Notes for mca i year students osmania university.docx

Python Notes for mca i year students osmania university.docx
80 ĐỀ THI THỬ TUYỂN SINH TIẾNG ANH VÀO 10 SỞ GD – ĐT THÀNH PHỐ HỒ CHÍ MINH NĂ...

80 ĐỀ THI THỬ TUYỂN SINH TIẾNG ANH VÀO 10 SỞ GD – ĐT THÀNH PHỐ HỒ CHÍ MINH NĂ...
Salient Features of India constitution especially power and functions

Salient Features of India constitution especially power and functions
ICT Role in 21st Century Education & its Challenges.pptx

ICT Role in 21st Century Education & its Challenges.pptx
Exploring_the_Narrative_Style_of_Amitav_Ghoshs_Gun_Island.pptx

Exploring_the_Narrative_Style_of_Amitav_Ghoshs_Gun_Island.pptx
UGC NET Paper 1 Mathematical Reasoning & Aptitude.pdf

UGC NET Paper 1 Mathematical Reasoning & Aptitude.pdf
ICT role in 21st century education and it's challenges.

ICT role in 21st century education and it's challenges.
21st_Century_Skills_Framework_Final_Presentation_2.pptx

21st_Century_Skills_Framework_Final_Presentation_2.pptx
HMCS Max Bernays Pre-Deployment Brief (May 2024).pptx

HMCS Max Bernays Pre-Deployment Brief (May 2024).pptx
Thomson atom model
- 1. Made by Olivia, Anna, Kaylee, and Andrew
- 2. ● Born December 18, 1856 in Cheetham Hill, England ● Died August 30 ,1940 in Cambridge, United Kingdom ● His brother was Frederick ● Thomson started his education in a private school ● Thomson had a lot of knowledge in science ● Thomson moved to Trinity College in Cambridge. ● Thomson married Rose Elizabeth Paget. JJ Thomson Background
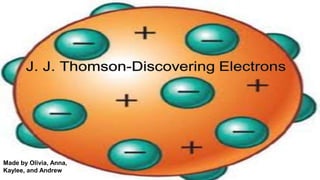
- 3. ● J.J Thomson discovered the electron in 1897 ● Made the plum pudding model of the atom ● In 1904 the discovery of the atomic nucleus ● Thomson’s model composed of electrons called corpuscles ● He figured out that you can divide atoms
- 4. ● Thomson’s model was not a solid but a neutral sphere ● Thomson’s model has positive and Negative charge ● His plum pudding model of an atomic structure proposed in the late 19th century
- 5. Cathode Ray Tubes ● Thomson found rays that were green and carried electricity, scientists named these tubes cathode tubes. ● Computers and TV contains cathode ray tubes. ● He discovered that cathode rays and the charge they deposited were intrinsically linked together. ● In the second experiment , he discovered that the charge in the cathode rays was negative. He deduced that the cathode rays were made up of negatively-charged particles.
- 6. Thomson’s Accomplishments ● He was awarded the Nobel Prize in the the Nobel Prize in Physics in 1906 for the discovery of the electron. ● He was knighted in 1908 and appointed to the Order of Merit in 1912
- 7. An electron is a is a particle with one negative charge. All atoms are made of a nucleus and electrons. Electrons are very small compared to the rest of the atom.electrons are extremely small compared to the rest of the atom and hold negative charges that balance the nucleus of an atom.The mass of an electron is 1,000 times smaller than the mass of a proton What is an Electron?