01-THE ATOM-TIMELINE FOR LEAVING CERTIFICATE.pptx
•Download as PPTX, PDF•
0 likes•2 views
History of the atom
Report
Share
Report
Share
Recommended
Recommended
More Related Content
Similar to 01-THE ATOM-TIMELINE FOR LEAVING CERTIFICATE.pptx
Similar to 01-THE ATOM-TIMELINE FOR LEAVING CERTIFICATE.pptx (20)
Recently uploaded
Recently uploaded (20)
Beyond_Borders_Understanding_Anime_and_Manga_Fandom_A_Comprehensive_Audience_...

Beyond_Borders_Understanding_Anime_and_Manga_Fandom_A_Comprehensive_Audience_...
Basic Civil Engineering first year Notes- Chapter 4 Building.pptx

Basic Civil Engineering first year Notes- Chapter 4 Building.pptx
This PowerPoint helps students to consider the concept of infinity.

This PowerPoint helps students to consider the concept of infinity.
NO1 Top Black Magic Specialist In Lahore Black magic In Pakistan Kala Ilam Ex...

NO1 Top Black Magic Specialist In Lahore Black magic In Pakistan Kala Ilam Ex...
Food safety_Challenges food safety laboratories_.pdf

Food safety_Challenges food safety laboratories_.pdf
Kodo Millet PPT made by Ghanshyam bairwa college of Agriculture kumher bhara...

Kodo Millet PPT made by Ghanshyam bairwa college of Agriculture kumher bhara...
General Principles of Intellectual Property: Concepts of Intellectual Proper...

General Principles of Intellectual Property: Concepts of Intellectual Proper...
HMCS Max Bernays Pre-Deployment Brief (May 2024).pptx

HMCS Max Bernays Pre-Deployment Brief (May 2024).pptx
01-THE ATOM-TIMELINE FOR LEAVING CERTIFICATE.pptx
- 3. SCIENTISTS John Dalton William Crookes J.J. Thomson (George Stoney) Ernest Rutherford Robert Millikan James Chadwick
- 4. Put forward an atomic theory based on experiments done with gases. This theory proposed that: 1. All matter is made up of tiny particles called atoms. 2. All atoms are indivisible. They cannot be broken down into simpler particles. 3. Atoms of the same element have identical mass and physical properties. 1808 John Dalton
- 5. 1870’s William Crookes – Cathode Rays Radiation coming from the cathode to the anode. Radiation caused the paddle wheel to move
- 6. 1897 J.J. Thomson – Discovery of the Electron Identified cathode rays as subatomic particles, and measured their charge to mass ratio e/m. Identified as electrons. (George Stoney is credited with the name electron)
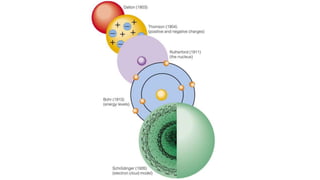
- 7. 1904 J.J. Thomson Plum pudding model for the atom. Mass of positively-charged material with electrons (small negative charges) scattered (embedded) in it.
- 8. 1909 Rutherford – Discovery of the Nucleus
- 9. 1909 Rutherford Some alpha particles were deflected at large angles A few alpha particles were reflected back along their original path Most alpha particles passed straight through undeflected Particles repelled as they passed close to a positive mass (nucleus). Particles collided with a small dense positive mass (nucleus). Atom is mainly made up of empty space. Note: This observation was not inconsistent with the ‘plum pudding’ Alpha Particles Helium nuclei Particles consisting of 2 protons and 2 neutrons.
- 10. 1911 Rutherford Discovery of the protons in the nuclei of various atoms. Different Elements to Gold (much lighter) The alpha particles where breaking up the nucleus to release positively charged particles (protons).
- 11. 1911 Robert Millikan Measured the charge on the electron using his oil drop experiment.
- 12. 1932 James Chadwick Discovery of the neutron. Neutrons