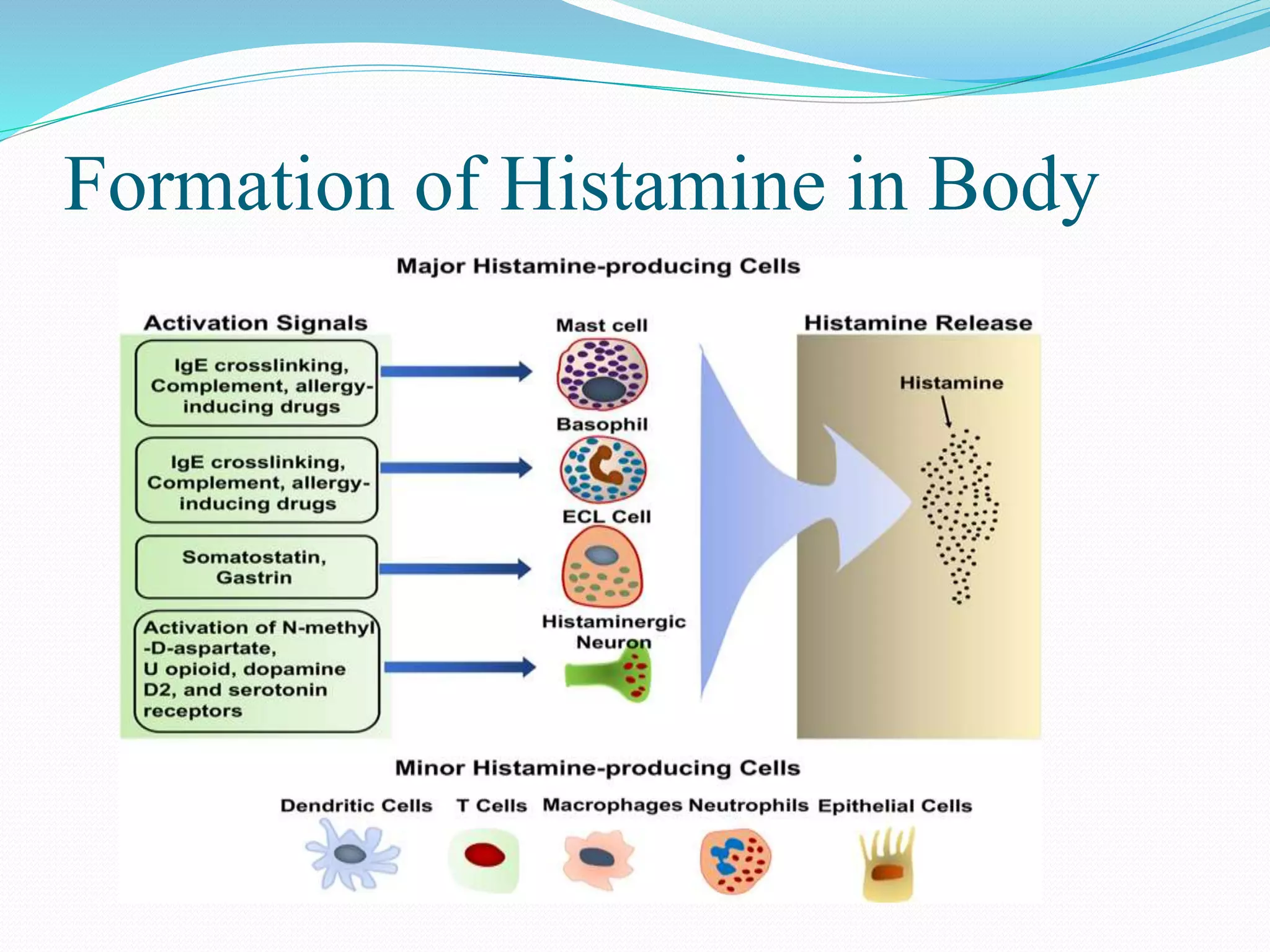
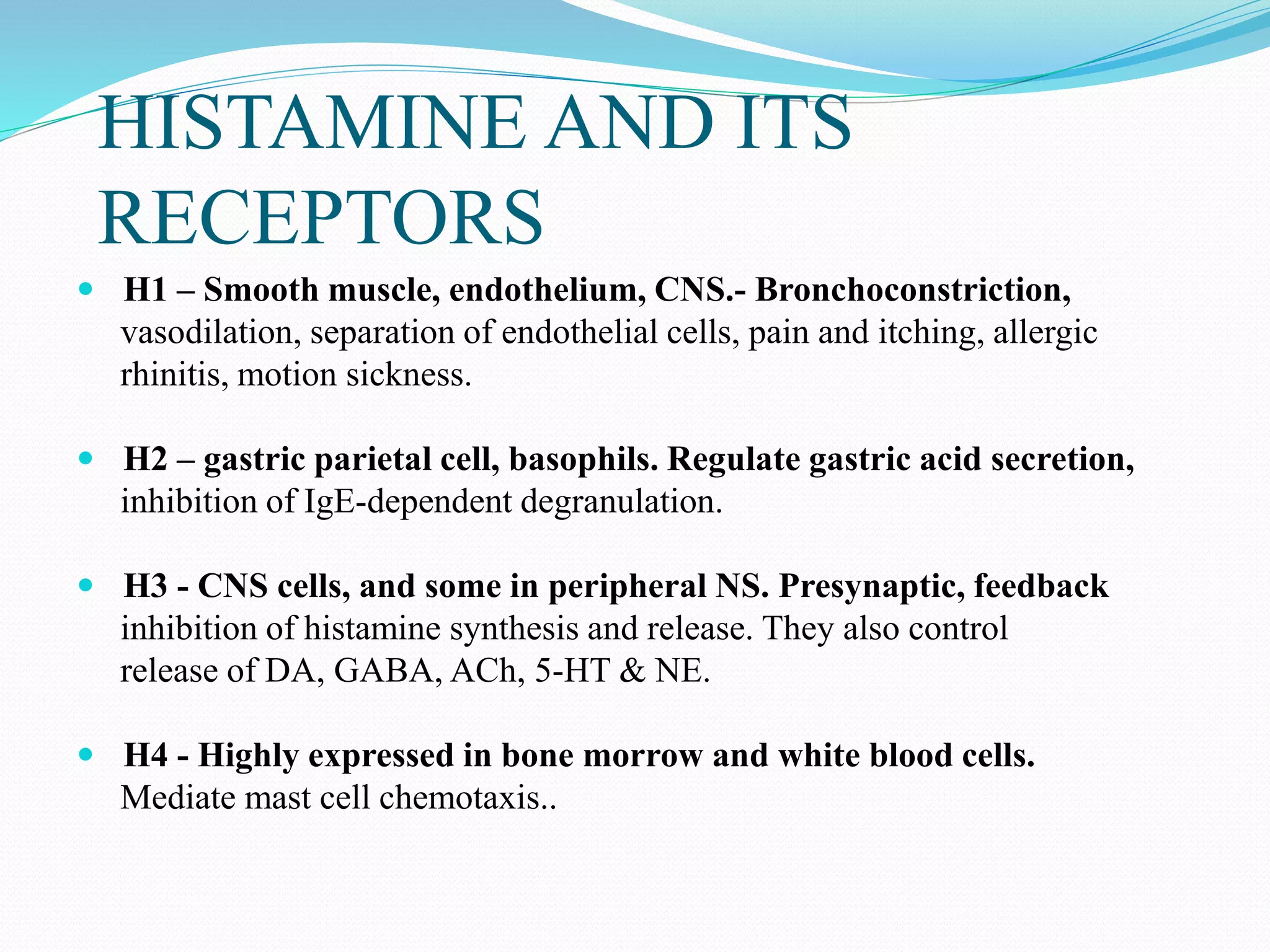
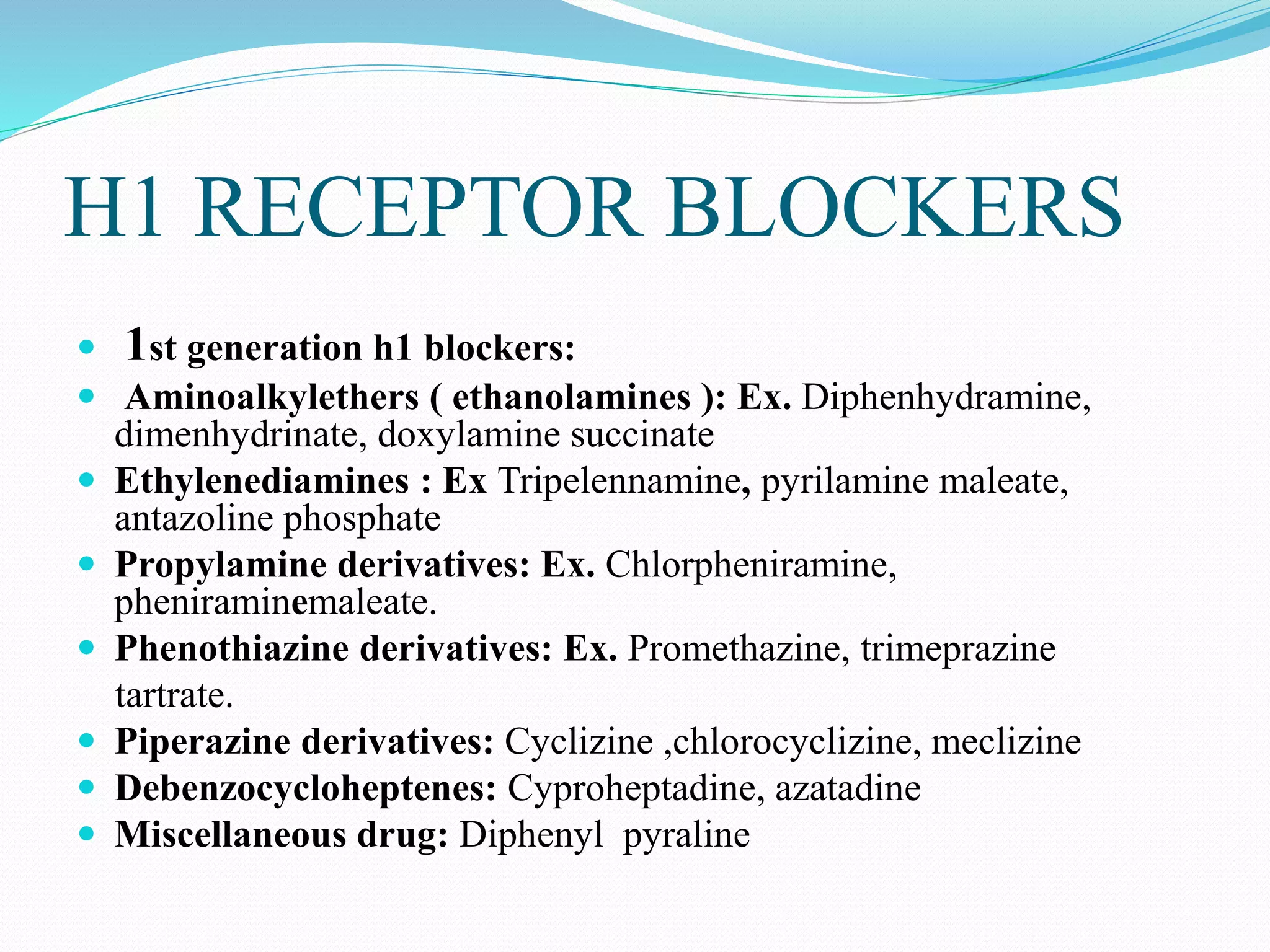
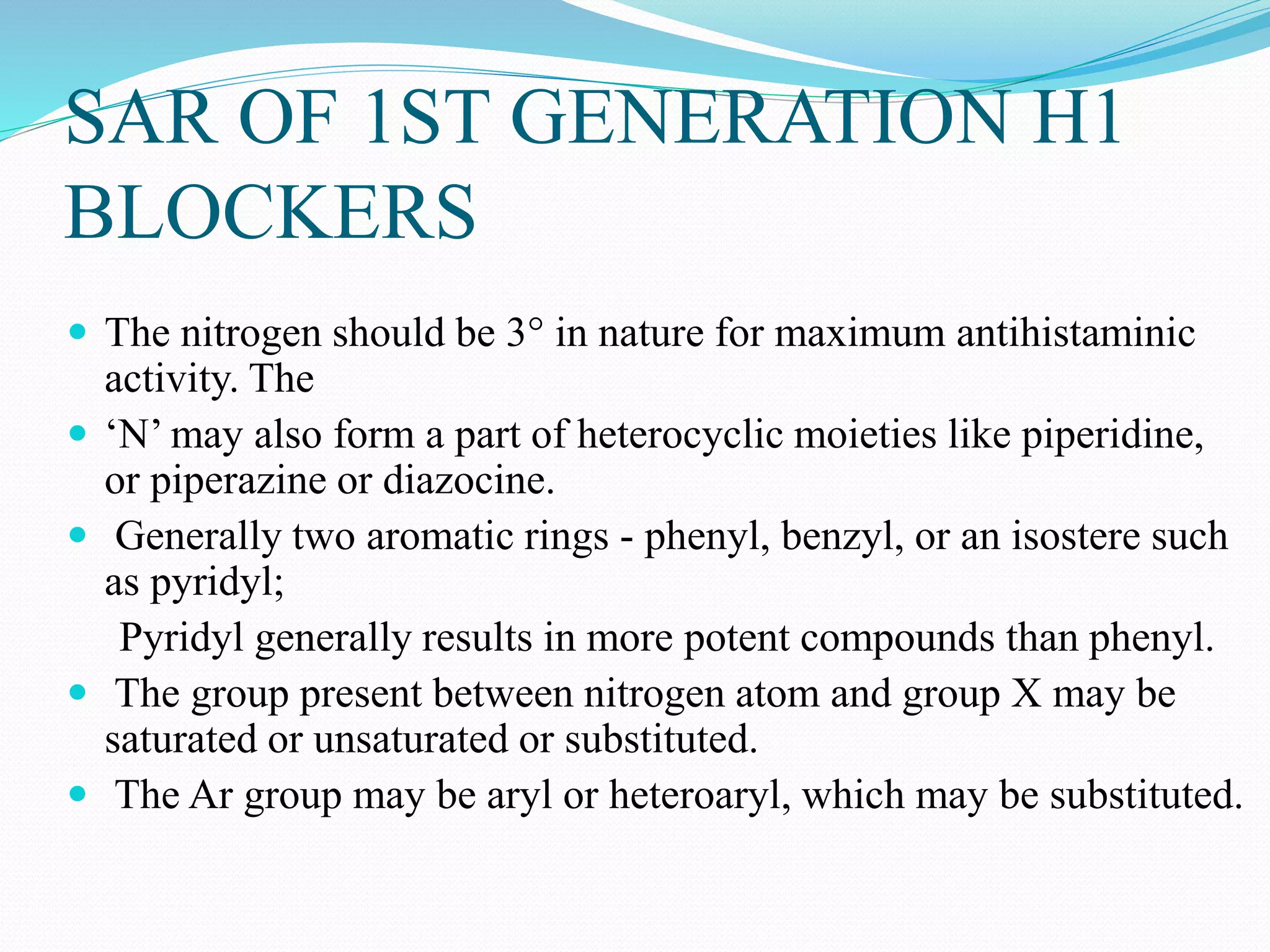
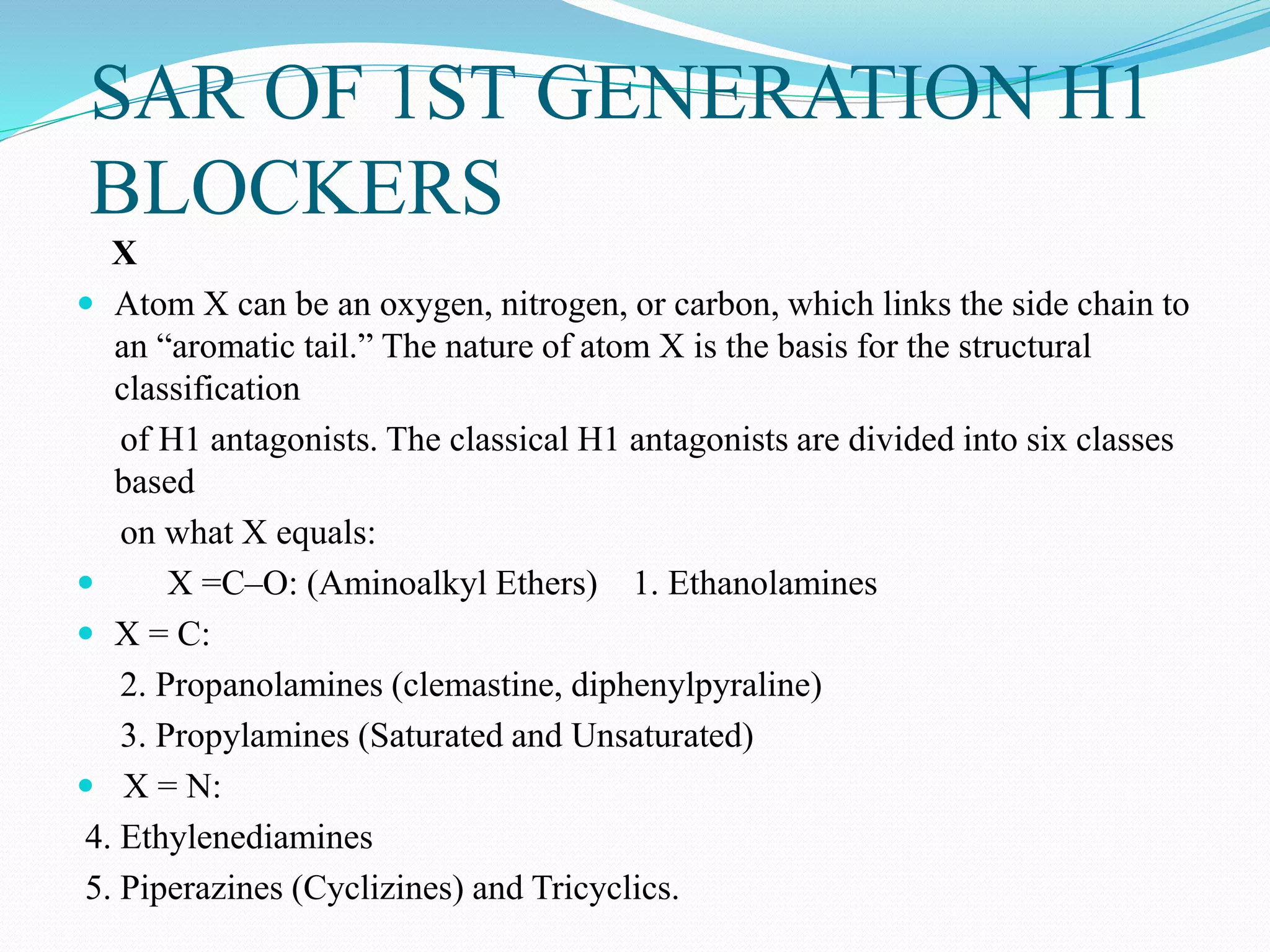
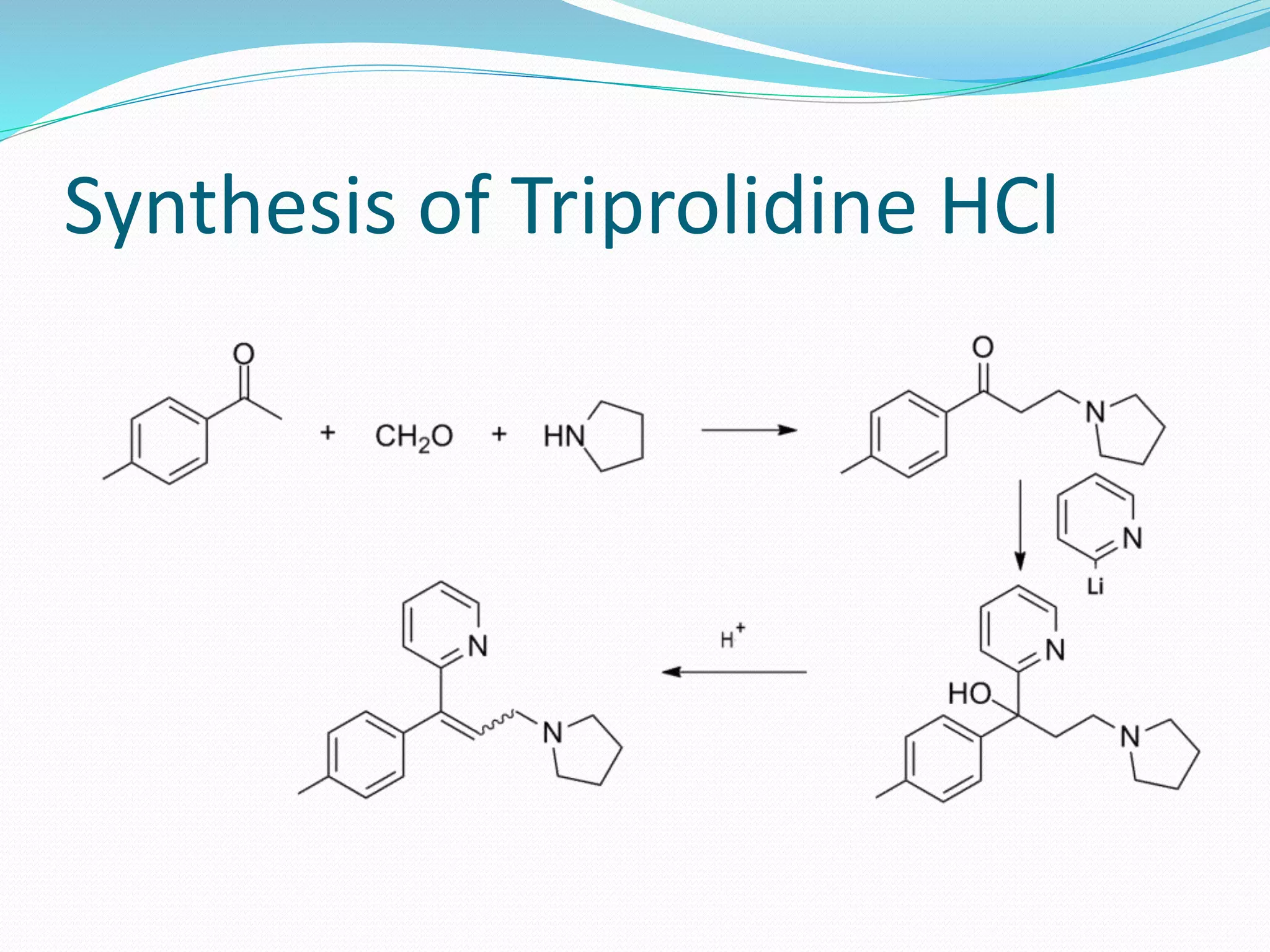
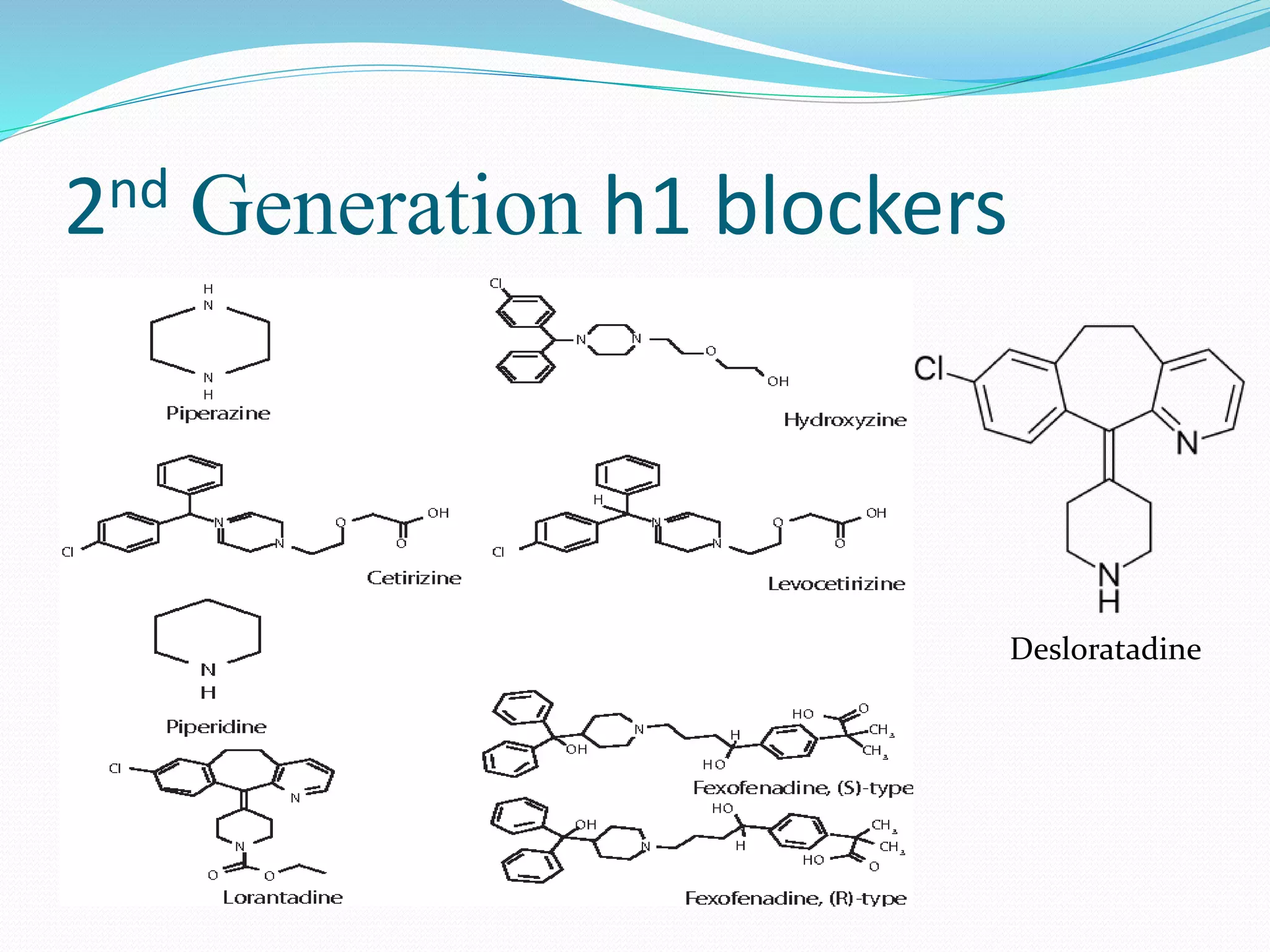
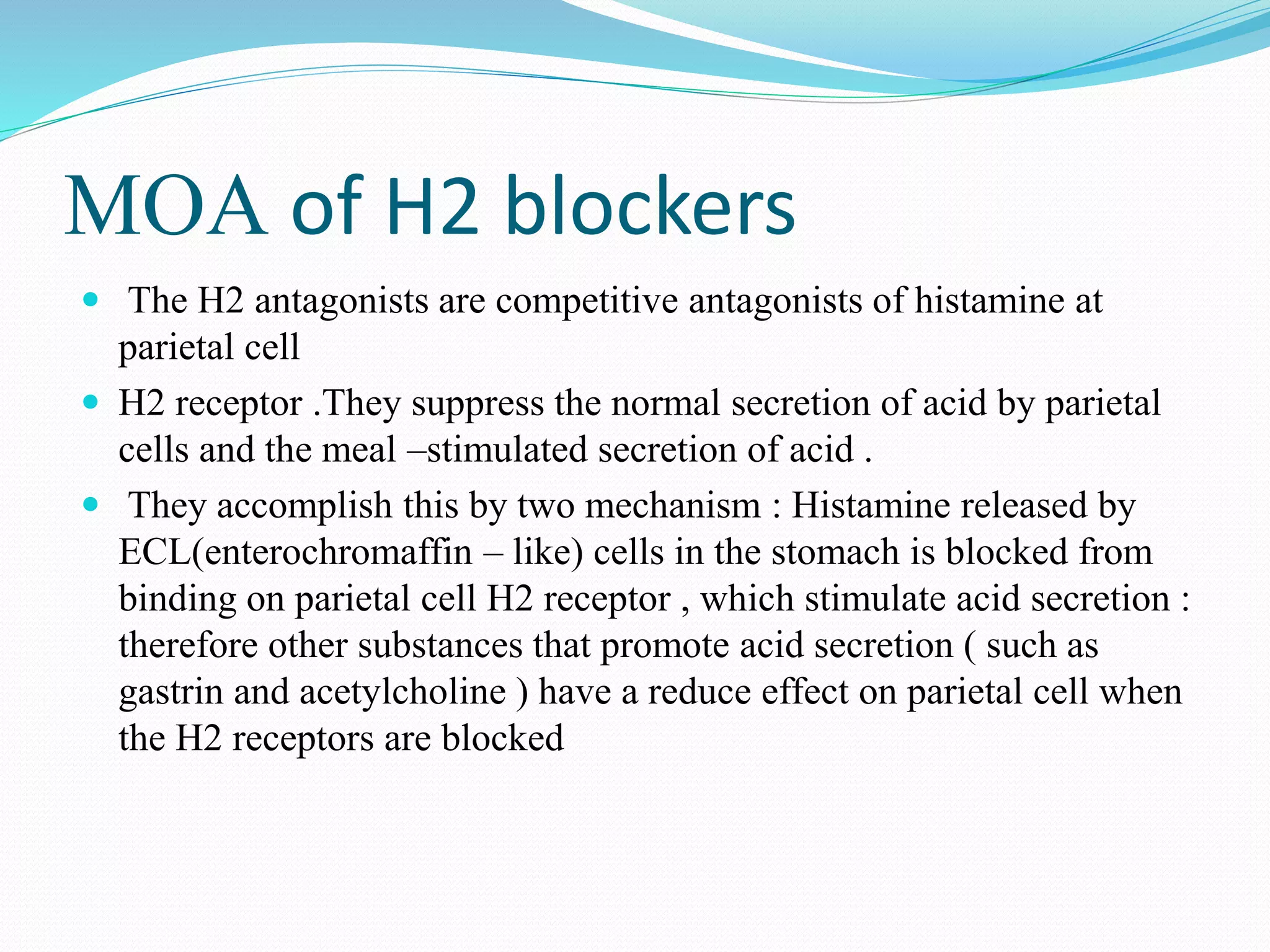
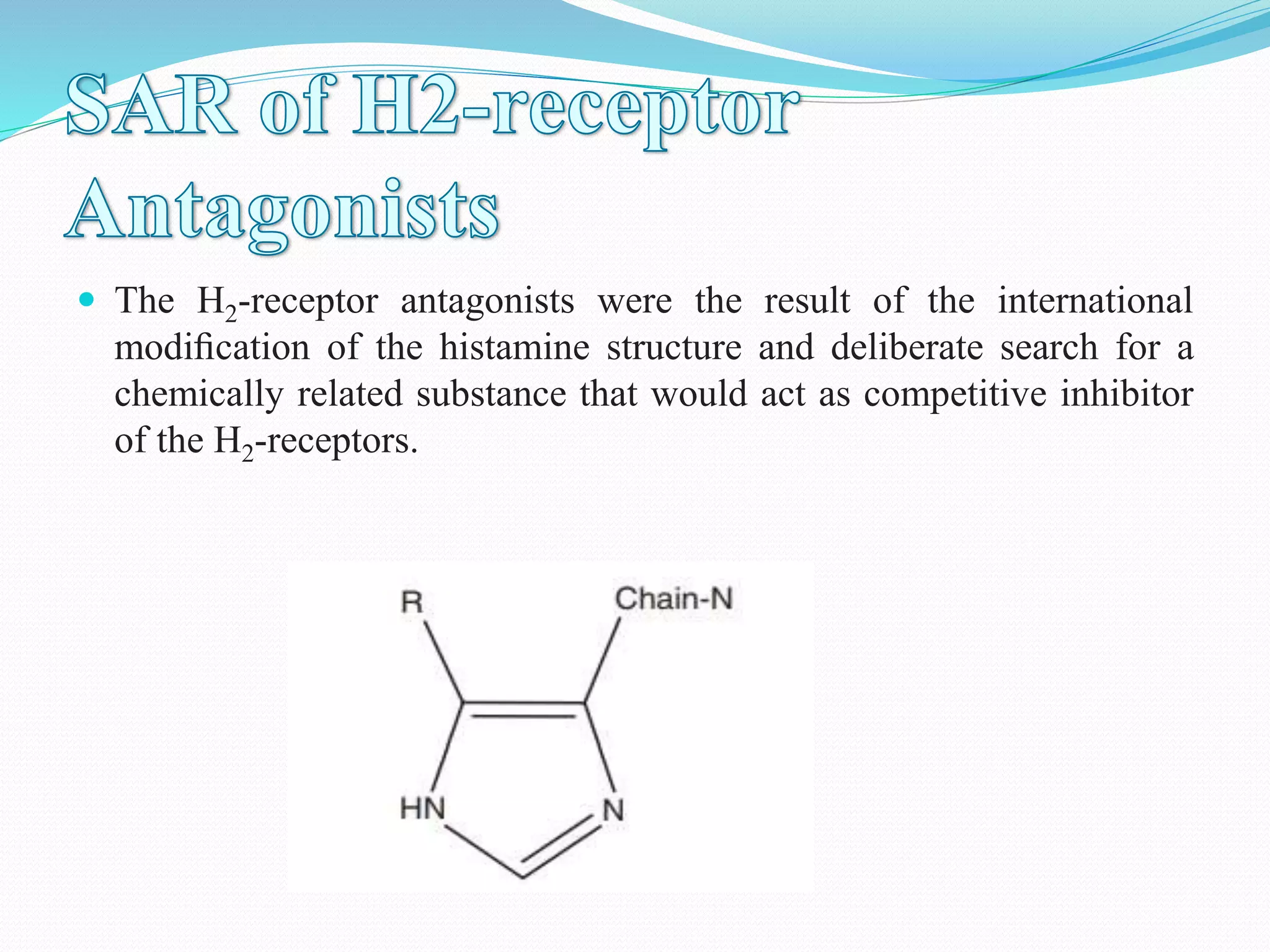
The document discusses histamine, its roles in allergic reactions, and its formation from histidine. It outlines the functions of histamine receptors (H1, H2, H3, and H4) and categorizes antihistamines into first and second generations, detailing their structures and uses. Additionally, it covers H2 receptor antagonists, their mechanisms of action, side effects, and therapeutic applications.