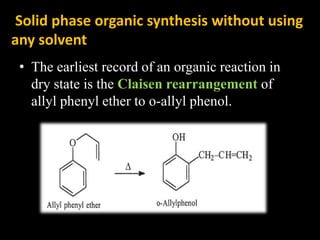
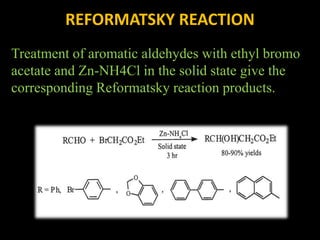
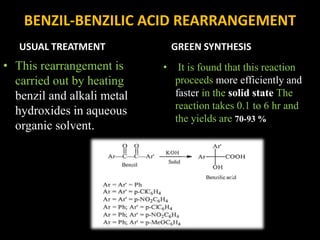
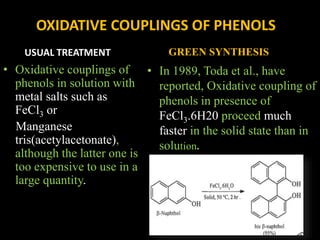
Solventless reactions have gained popularity in green chemistry as they avoid using toxic organic solvents. Some key advantages are that they are more efficient and selective than solvent-based reactions. They also reduce costs by saving on solvents, simplify purification without needing to remove solvent, and are more environmentally friendly. Common solventless reactions include halogenation, Michael additions, aldol condensations, and oxidative couplings of phenols. While homogeneous reactants are required and solvents may still be used during workup, solventless reactions provide an important technique for greener organic synthesis.















![• Synthesis of
Azomethines
• Dimerisation of [60]
fullerene
• Synthesis of Homoallylic
Alcohols
• Synthesis of Thio
carbonylimidazolide
Derivatives](https://image.slidesharecdn.com/solventlessreaction-170917070306/85/Solventless-reaction-in-green-chemistry-20-320.jpg)



