Module No. 35
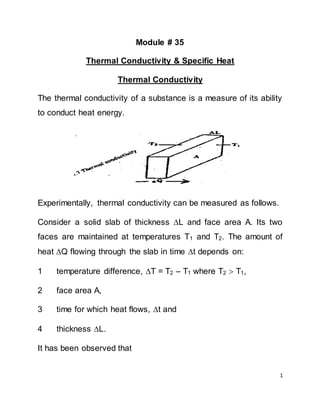
- 1. 1 Module # 35 Thermal Conductivity & Specific Heat Thermal Conductivity The thermal conductivity of a substance is a measure of its ability to conduct heat energy. Experimentally, thermal conductivity can be measured as follows. Consider a solid slab of thickness L and face area A. Its two faces are maintained at temperatures T1 and T2. The amount of heat Q flowing through the slab in time t depends on: 1 temperature difference, T = T2 – T1 where T2 T1, 2 face area A, 3 time for which heat flows, t and 4 thickness L. It has been observed that
- 2. 2 Q A, Q T, Q t and Q 1/L A x T x t A x T x t Q ---------------- OR Q = K ----------------- [1] L L Where, K is a constant of proportionality called thermal conductivity. Its value depends on the material of the slab and is a measure of the thermal conductivity of the material. If A = 1m2 L = 1m T = 1°C and t = 1 Sec then, by putting these values in Eq. [1] we get,
- 3. 3 Q = K Thus, the thermal conductivity can be defined as "The amount of heat conducted for one second through a meter cube of the substance whose two opposite faces are maintained at a temperature difference of 1°C." K is large for metals and small for non-metallic solids, liquids and gases. Thermal Efficiency It is defined as the ratio of the heat actually utilized to the total heat produced. Consider the case of the electric kettle used for boiling water. Out of the total heat produced (i) some goes to heat the apparatus itself i.e. kettle (ii) some is lost in the air due to radiation. The remaining (iii) is utilized for heating the water. Out of these, the heat utilized for useful purpose is that in (iii). Hence, thermal efficiency of this electric apparatus is the ratio of the heat utilized for heating the water to the total heat produced. Remember that 1 K calorie = 4187 joules 1 Ib. = 0.4536 Kg
- 4. 4 1 B.T.U = 0.252. Kcal 1°F = 5/9°C 1 gallon = 10 Ibs. Thermal Engineering The field of engineering science which deals with the applications of thermodynamics and its laws to work producing and work absorbing devices, in order to understand their performance, is known as thermal Engineering. Thermometric Properties of Matter A thermometric property of matter is that property of matter which changes uniformly with change of temperature. Examples of thermometric properties are: (1) Expansion or Change of Volume The volume of a gas, liquid and solid changes with temperature. Nearly, all materials expand on heating and contract on cooling. For example, iron is longer when it is hot than when it is cold. (2) Change of Color The color of matter changes with change of temperature. At very high temperatures, solids become red. At still higher
- 5. 5 temperatures, iron and some other solids turn orange and then white. (3) Change of Electrical Resistance Electric resistance of a conductor is directly proportional to its temperature. It means that higher the temperature of the wire, the greater is its resistance. This is known as thermometric property of conductor. Thus, the resistance of a metallic wire increases on heating and decreases on cooling. (4) Change of Physical State Change of temperature also affects the physical state of a material. For example, water at low temperature is ice (a solid), at a higher temperature, it is liquid, and still at higher temperature; it is steam (a gas). (5) Heat Radiation Material objects emit heat or thermal radiation when heated. The intensity of these radiations increases with rise in temperature. Mechanical Theory of Heat The first clear experimental observations showing that caloric cannot be conserved were made at the end of the eighteenth century by Count Rumford, a German army engineer. He supervised the boring of huge cannon barrels in the Munich
- 6. 6 Arsenal. Because of the heat generated by the boring tool, water was used for cooling. It had to be replaced continually because it boiled away during the boring. According to the caloric theory, as the metal from the bore was cut into small chips, its ability to retain caloric was decreased. Therefore, it released caloric to the water, heating it and causing it to boil. Rumford noticed, however, that even when the drill was too dull to cut the metal, the water still boiled away as long as the drill was turned. Rumford also realized that this process would go on indefinitely and produce limitless amount of heat. This was not consistent with the idea that heat is a substance and that only a finite amount of it could be contained within an object. Rumford, therefore, rejected the caloric theory and correctly guessed that heat was only a form of energy which when disappears, definite proportion of heat is produced. Therefore, he concluded that heat is not the flow of caloric but it is a form of energy which is produced by friction or by mechanical work. The idea was supported by Joule and he proved by experiment that, 4.2 Joule of work produces 1 calorie heat. Therefore, 4.2 J is called mechanical Equivalent of heat. Mechanical Equivalent of Heat Mechanical equivalent of heat is equal to the amount of work
- 7. 7 required to produce one unit of heat. Its value is 778 ft-lb/BTU and 427 Kg-m/Kcal. It may be defined as the number of works unit which, when completely converted into heat, furnish 1 unit of heat. It has been found that J is equal to 4.2 joules/calorie. (1 joule = 107 ergs). Total Heat or Enthalpy of Steam It is the amount of heat absorbed by water from freezing point to saturation temperature plus the heat absorbed during evaporation. Total heat or enthalpy of steam = sensible heat + Latent heat It is denoted by (H) and its value for dry saturated steam may be read directly from steam tables. Specific Heat The quantity of heat required to raise the temperature of a body of mass 1kg by 1k is called specific heat. It can be expressed mathematically as Q C = ----------- m T
- 8. 8 Where Q is the amount of heat which causes a change T in the temperature of the substance having mass ‘m’. Unit of Specific Heat The unit of specific heat is Joule per kilogram per Kelvin. OR Joule per kilogram per degree centigrade. Specific heat does not depend upon shape, volume or mass of the body, but only on the material which makes the body. Relation between Specific Heats The specific heats CP & Cv of a perfect gas are assumed to remain constant at all temperatures, but for real gases they increase considerably at high temperatures. For any particular gas the specific heat at constant pressure is always greater than specific heat at constant volume and the ratio of the two remains constant. The constant is denoted by γ. In other word, γ = Cp/Cv For air
- 9. 9 Cv = 0.17 kcal/kg-°k Cp = 0.24 kcal/kg-°k And in SI unit For air Cv = 2.7128 kJ/Kg-°k CP = 1 KJ/kg-°k Another relationship between specific heats and characteristic gas constant is CP-Cv = R/J R is gas constant, whose value is taken as 0.287 KJ/Kg-°k and J is mechanical heat equivalent. Specific Heat of Water is higher while that of Earth is Low Higher specific heat of water effects the climate i.e. climate of areas situated around the lakes or sea shores is always mild. During the daytime, the earth because of low specific heat gets heated up quickly. Therefore, the air overland becomes warmer and rises up. Thus, cold air from sea moves towards the land and
- 10. 10 lowers the temperature on land. This movement of air from sea to land is called sea breeze. Similarly, in the night, the land quickly cools down, but, the air over water remains warmer. So, this breeze moves from land to sea. These breezes keep temperature of the area milder. Specific Heat Capacity The specific heat capacity of a substance is defined as the heat required to raise the temperature of unit mass of it through 1K. The SI unit of specific heat capacity is the joule per Kilogram Kelvin (J/Kg K) or KJ/Kg or MJ/Kg. Specific Heat of a Gas The specific heat of a substance may be broadly defined as the amount of heat required to raise the temperature of its unit mass through 1°C. All the liquids and solids have one specific heat only. But a gas can have any number of specific heats (lying between zero and infinity) depending upon the conditions, under which it is heated. The following two types of specific heats of a gas are important from the subject point of view. 1. Specific heat at constant volume and 2. Specific heat at constant pressure.
- 11. 11 Specific Heat of a Gas at Constant Pressure (CP) Specific heat of a gas at constant pressure is defined as the amount of heat required to raise the temperature of a unit mass of a gas through 1°C. It is generally denoted by CP. Thus, it is the amount of heat required to raise the temperature of a unit mass of a gas through unit degree when the pressure is kept constant. If m kg of a gas is heated at constant pressure from initial Temperature TI to final Temperature T2, then heat supplied is Q = m CP (T2-T1) Specific Heat of a Gas at Constant Volume (Cv) Specific heat of a gas at constant volume is defined as the amount of heat required to raise the temperature of a unit mass of a gas through 1°C when it is heated at constant volume. It is denoted by Cv. Thus, it is the quantity of heat required to raise the temperature of a unit mass of a gas through unit degree when the volume is kept constant. If m kg of a gas is heated at constant volume from initial temperature T1 to final temperature T2, then heat supplied is
- 12. 12 Q = m Cv (T2-T1) Latent Heat The heat energy involved in a change of state is called Latent heat. Latent Heat of Melting The quantity of heat required to transform one kilogram of solid completely into liquid at its melting point is called latent heat of melting or fusion. Latent heat of ice is 3.36 x 105 J/Kg. It means that 3.36 x 105 J of heat is required to transform one Kg of ice into water at 0°C. When a solid is heated, its molecules vibrate vigorously because their kinetic energy is increased. While on further heating, the vibrations become so vigorous that they overcome the forces of attraction between the molecules. So, these molecules start moving apart and the solid starts melting. During this process, the heat supplied to the solid is not indicated by rise in thermometer temperature. Because this heat is used to overcome the forces of attraction of molecules, therefore, temperature does not rise. Latent Heat of Vaporization It is the amount of heat absorbed to evaporate 1 kg of water at its boiling point, without change of temperature. It is denoted by L,
- 13. 13 and its value depends upon the pressure. The latent heat of vaporization of water or latent heat of steam is 537 kcal/kg at atmospheric pressure. It has been experimentally found that the value of L decreases as the pressure increases. Latent Heat of Vaporization or Boiling (Steam) The amount of heat required to transform the mass of one kg of liquid completely into gas at its boiling point is called the latent heat of boiling or vaporization. The latent heat of water is 2.26 x 106 J/Kg. It means that one Kg of water requires 2.26 x 106 J to change into gas at 100o C. When a liquid is heated, its temperature rises and becomes equal to the boiling point. At this temperature, the movement of molecules becomes vigorous. This fast motion overcomes the forces of attraction between molecules. So, some of these molecules escape from the surface of liquid and converts the liquid into gas. During this process, heat absorbed is used to change the liquid into gas and, therefore, during this process, temperature does not rise. Specific Latent Heat of Vaporization The specific latent heat of vaporization of a substance is the
- 14. 14 quantity of heat required to change unit mass of the substance from the liquid to the vapor state without change of temperature. The SI unit of specific latent heat of vaporization is the joule per kilogram (J/kg). Specific Latent Heat of Fusion The specific latent heat of fusion of a substance is the quantity of heat required to convert unit mass of the substance from the solid to the liquid state without change of temperature. The SI units of specific latent heat of fusion are J/Kg or KJ/Kg or MJ/kg.
