Hydrogenation
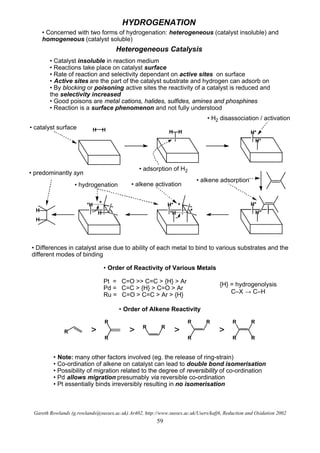
- 1. Gareth Rowlands (g.rowlands@sussex.ac.uk) Ar402, http://www.sussex.ac.uk/Users/kafj6, Reduction and Oxidation 2002 59 * HYDROGENATION • Concerned with two forms of hydrogenation: heterogeneous (catalyst insoluble) and homogeneous (catalyst soluble) Heterogeneous Catalysis • Catalyst insoluble in reaction medium • Reactions take place on catalyst surface • Rate of reaction and selectivity dependant on active sites on surface • Active sites are the part of the catalyst substrate and hydrogen can adsorb on • By blocking or poisoning active sites the reactivity of a catalyst is reduced and the selectivity increased • Good poisons are metal cations, halides, sulfides, amines and phosphines • Reaction is a surface phenomenon and not fully understood H H H H H* H* H* H* **H H***H H H H • catalyst surface • adsorption of H2 • H2 disassociation / activation • alkene adsorption • alkene activation• hydrogenation • predominantly syn • Differences in catalyst arise due to ability of each metal to bind to various substrates and the different modes of binding • Order of Reactivity of Various Metals Pt = C=O >> C=C > {H} > Ar Pd = C=C > {H} > C=O > Ar Ru = C=O > C=C > Ar > {H} {H} = hydrogenolysis C–X → C–H • Order of Alkene Reactivity R R R R R R R R R R R R > > > > • Note: many other factors involved (eg. the release of ring-strain) • Co-ordination of alkene on catalyst can lead to double bond isomerisation • Possibility of migration related to the degree of reversibility of co-ordination • Pd allows migration presumably via reversible co-ordination • Pt essentially binds irreversibly resulting in no isomerisation
- 2. Gareth Rowlands (g.rowlands@sussex.ac.uk) Ar402, http://www.sussex.ac.uk/Users/kafj6, Reduction and Oxidation 2002 60 Stereoselectivity • Mechanism (vide supra) indicates the addition is predominanly syn • As substrate and hydrogen are both bound to surface addition occurs from the least hindered face as more readily binds to surface) • Problem: isomerisation can lead to anti addition • Problem: predicting which face will bind to surface not as simple as above statement suggests • Haptophilicity is the ability of a functional group to anchor to the surface and direct which face of alkene co-ordinates H H H H • normally hydrogen adds from least hindered side • hydrogen adds from opposite face • functional group • functional group attracted to surface Alkynes O • Lindlar catalyst (Pd / CaCO3 / PbO) optimum catalyst to prevent over-reduction and cis / trans isomerisation O H2, Lindlar, BuOH, rt 95 % • syn addition Heteroatom Hydrogenations Carbonyl Moiety • Can be hydrogenated • Stereoselectivity hard to predict so prefer hydride reagents • Platinum reagents preferred as C=O faster than C=C (vide supra) especially when poisoned N H HO CO2Et O H2, PtO2, AcOH, H2O N H HO CO2Et OH • Order of carbonyl reduction R Cl O R R(H) O R O R O O R OR O R OH O R NH2 O > > > > >
- 3. Gareth Rowlands (g.rowlands@sussex.ac.uk) Ar402, http://www.sussex.ac.uk/Users/kafj6, Reduction and Oxidation 2002 61 Nitriles N Bn C OBnBnO OBn BocHN N H2, Pd(OH)2 / C, MeOH N H OHHO OH BocHN NH2 Nitro Group C4H11 OO N O O O ( )3 C4H11 OO NH2 O ( )3 N H C4H11 OO ( )3 1. H2, Pd / C 2. (CO2H)2 Azides N N3 Ph O MeO2C H2. 5 % Pd / C N H2N Ph O MeO2C
- 4. Gareth Rowlands (g.rowlands@sussex.ac.uk) Ar402, http://www.sussex.ac.uk/Users/kafj6, Reduction and Oxidation 2002 62 Homogeneous Catalyst • Soluble in reaction medium • Mechanisms much better understood • Advantages: mild conditions (non-polar solvents which dissolve H2 better) • Advantages: less catalyst required (each molecule is available for reaction and not just surface) • Advantages: improved or complimentary selectivity (far more predictable) • Advantages: directed hydrogenations • Advantages: asymmetric hydrogenations Alkene Hydrogenation • 2 main types of homogeneous catalysts: dihydride and monohydride catalysts Dihydride Catalysts LnM + H2 H LnM H • Examples: Wilkinson's Catalyst ClRh(PPh3)3 (hydrogen adds prior to substrate) Crabtree's Catalyst [Ir(COD)(PCy3)(pyr)]+ PF6 – (substrate adds before H2) General Mechanism LnM H MLnMLn H LnM H LnM H LnM H HH HH H2 H2 reductive elimination reductive elimination • oxidative cis addition Wilkinson's catalyst Crabtree's catalyst
- 5. Gareth Rowlands (g.rowlands@sussex.ac.uk) Ar402, http://www.sussex.ac.uk/Users/kafj6, Reduction and Oxidation 2002 63 Monohydride Catalysts • LnM–H • Examples: HRu(Cl)(PPh3)3 Cp2TiH LnM H LnM H LnM H Ln M HHH LnM H HH 1,2-insertion cis-addition reductive elimination Wilkinson's Catalysis • Very well studied Cl Rh P P P S S Cl Rh P S P S S Cl Rh P H P H S Cl Rh P H P H R3 R1 R R2 Cl Rh P P H S R1 R H R3 R2 HH R3 R1 R R2 R3 R1 R R2 H2 RDS –P Rh+1 Rh+3 Rh+3 • oxidative addition • reductive elimination H2 oxidative addition • catalytic species • metal centre oxidised • insertion • S = solvent or vacant site • very fast; no isomerisation
- 6. Gareth Rowlands (g.rowlands@sussex.ac.uk) Ar402, http://www.sussex.ac.uk/Users/kafj6, Reduction and Oxidation 2002 64 Selectivity Ar R ( )n = 1,2 R R1 R R1 R R1 R R1 R2 > > > > >= • Like heterogeneous catalysts there is a strong steric selectivity for the least hindered alkenes O PO C5H11 (CH2)3CO2R OP H H ClRh(PPh3)3, H2 O PO C5H11 (CH2)3CO2R OP H H Stereoselectivity • As indicated in the mechanism reductive elimination is fast so no isomerisation can occur and syn addition results Ph H OMe H ClRh(PPh3)3, D2 OMePh HH DD • Like heterogeneous catalysts, hydrogenation occurs from the least hindered face O iPr ClRh(PPh3)3, H2 O iPr • less substituted alkene • addition from least hindered side O TrO OMe ClRh(PPh3)3, H2 O TrO OMe Functional Group Compatibilty • Compatible with most functional groups • Aldehydes often undergo decarbonylation N Cbz O N Cbz ClRh(PPh3)3 95 %
- 7. Gareth Rowlands (g.rowlands@sussex.ac.uk) Ar402, http://www.sussex.ac.uk/Users/kafj6, Reduction and Oxidation 2002 65 M M Directed Hydrogenation • A hydroxyl group in the substrate can displace a ligand from the catalyst resulting in directed hydrogenation • This can reverse normal selectivity HO O HO O H N Ir Cy3P Crabtree's catalyst H2 24 : 1 • same face • Crabtree's catalyst much more reactive than Wilkinson's; so good for hindered alkenes • Crabtree's catalyst gives superior directing effect for cyclic substrates • For acyclic substrates use Wilkinson's catalyst • If alkene isomerisation a problem use Wilkinson's catalyst at elevated pressure R OH H OH R H H L L R H H OH H L L R OH R OH M H R OH H L L M H OH H R L L R OH vs vs • disfavoured due to steric interactions anti syn • Note: only get stereocontrol if isomerisation is surpressed ASYMMETRIC HYDROGENATION • Many asymmetric variants have now been developed • Diphosphine ligands are very common Ph CO2Me NHCOMe + H2 + P Rh P S S PhAr Ph MeO Ph NHCOMe H CO2Me> 95 % e.e.
- 8. Gareth Rowlands (g.rowlands@sussex.ac.uk) Ar402, http://www.sussex.ac.uk/Users/kafj6, Reduction and Oxidation 2002 66 Rh Mechanism H N CO2Me O PhP P Rh H NMeO2C OPh P P Rh H N CO2Me OPhP H H P Rh H P O P S NH Ph CO2Me Rh H NMeO2C OPh P H H P Rh H P O P SHN Ph MeO2C Ph H NHCOMe CO2Me Ph H MeOCHN MeO2C Ph CO2Me NHCOMe P Rh P S S PhAr ArPh + fast fast H2 slow RDS kmajor H2 slow RDS kminor minormajor majorminor kminor : kmajor 573 : 1 • most stable complex • minor complex reacts much faster • the major product comes from the minor complex • Note: Substrate and metal must be complexed to get good e.e.
- 9. Gareth Rowlands (g.rowlands@sussex.ac.uk) Ar402, http://www.sussex.ac.uk/Users/kafj6, Reduction and Oxidation 2002 67 Non-Co-ordinated Asymmetric Catalysts • Catalysts that do not require co-ordination to the substrate to give good e.e.s still uncommon • They offer the advantage of greater structural variety • One example is: P N O tBuIr Ph Ph BARF Ph MeO 3 mol% cat., H2 50 bar 99 % 98 % e.e. Ph MeO BARF = tetrakis{3,5-trifluoromethyl}phenyl borate Monohydride Catalyst • Provides a second example TiX X X2 = 1,1'-binaphth-2,2'-diolate Ti H NR N H R 1. BuLi 2. PhSiH3 H2 (80-500 psi) 68-89 % 95-99 % e.e. Ti H Ti HN R Ti HN R Ti HN R H H vs N H R Mechanism • R group in space • R group clashes with ligand • concerted 4-centre cleavage of N–Ti
- 10. Gareth Rowlands (g.rowlands@sussex.ac.uk) Ar402, http://www.sussex.ac.uk/Users/kafj6, Reduction and Oxidation 2002 68 R Transfer Hydrogenation O OH + OH O + N ClN HPh Ph Ts Ru • free NH crucial • Mechanism is given in the Oxidation Section of this course • Problem: the reaction is reversible (hence the oxidation) • If formic acid / triethyl amine is used as the reductant reaction irreversible N H N H O O H+ N H NH + O C O cat. Et3N • gives off CO2 hence irreversible Hydrogenolysis R X R H H2 O I OMe H I H2, Ni[R] O OMe H • Used to remove various functional groups • Or protecting groups O O R OPh O H2, Pd / C O O R OH O Easiest Hardest RCOCl RCHO RNO2 RNH2 RC≡CR' RCH=CHR' RCHO RCH2OH RCH=CHR' RCH2CH2R' RCOR' RCHOHR' ArCH2OR ArCH3 + ROH RC≡N RCH2NH2 RCO2R' RCH2OH + R'OH Ease of reduction of functional groups towards catalytic hydrogenation • note how far down benzyl group is • Note: different catalysts have different propensities for functional groups so this is only a rough order