Option C Nernst Equation, Voltaic Cell and Concentration Cell
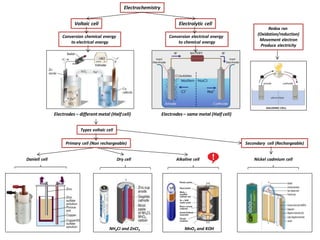
- 1. Types voltaic cell Conversion electrical energy to chemical energy Electrochemistry Electrolytic cellVoltaic cell NH4CI and ZnCI2 Redox rxn (Oxidation/reduction) Movement electron Produce electricity Conversion chemical energy to electrical energy Electrodes – different metal (Half cell) Electrodes – same metal (Half cell) Daniell cell Alkaline cellDry cell Nickel cadmium cell Primary cell (Non rechargeable) MnO2 and KOH Secondary cell (Rechargeable)
- 2. Current– measured Amperesor Coulombs per second 1A = 1 Coulomb charge pass througha point in 1 s = 1C/s 1 Coulomb charge (elec) = 6.28 x 10 18 elec passing in 1 s 1 elec/proton carry charge of – 1.6 x 10 -19 C ( very small) 6.28 x 10 18 elec carry charge of - 1 C Electric current Flow electric charges (elec, -ve) From High to low electric potential Potential Diff – measure with ammeter ond electron ond Coulomb A sec.1 .1028.6 sec1 1 1 18 Current Electric Current – movingcharges in solid wire or solution Flow of charges - - - Solid/WireSolution/Electrolyte Electron move in random No current flow cause No potential difference Electrons & Protons - - + + 1A = 6.28 x 1018 e 1 s Potential Difference across wire Electron move in one direction Current flow +ve ions -ve ions (cations) (anions) Potential Diff applied/Battery ItQ t = Time/ s Find amt charges pass through if Current is 2.ooA, time is 15 min ItQ Current flow Q = Amt Charges/ C I = Current/ A CQ 1800601500.2
- 3. Electric Potential C J Volt 1 1 -Measured in Volt with Voltmeter - 1 V = 1 Joule energy released when 1 Coulomb charge pass through 1 point - 1 V = 1 J/C V = Potential Diff I = Current R = Resistance Potential diff bet 2 points is 1 V ↓ 1 J energy released when 1 C charge passes through Voltmeter across 1Volt 1 V + - 1 Ω 2 Ω Charges (-ve) flow down A R V I RIV 2 3 6 VV RIV 212 - + - + VV RIV 422 Total current Potential Diff(PD)vs Current PD = Water Pressure PD = 1.5V – 1.5J energy released 1C charge flow down PD – cause charge flow = CURRENT Potential Diff(PD)vs Current 1.5V = 1.5J/C A DElectric potential/PD/Voltage = Electric Pressure = Volt Electric Current = Charge flow = Amp Electric Potential Energy = Work done to bring a charge to a point = Joule Voltage NOT same as energy, Voltage = energy/charge Battery lift charges, Q to higher potential Potential Energy bet 2 terminals in battery stored as chemical energy 2A 2A Potential Diff/VoltagePotential Diff/Voltage
- 4. EMF vs PD V = Potential Diff I = Current R = Resistance Max potential diff bet two electrodes of battery source. + - 1 Ω 2 Ω A R V I RIV 2 3 6 VV RIV 212 VV RIV 422 Total current Current flow Circuit complete Circuit complete ↓ Current flow ↓ Internal resistance (battery - 1Ω) ↓ Terminal PD = 8V (Voltage drop) Potential Diff/Voltage in Volt Symbol for EMF = E / ℰ No Current flow in circuit EMF (Electromotive Force) Volt Battery = EMF = 9V 9 Volt ).(9 currentnoVEMFV IRV EMF Internal resistance Ir Place voltmeter across – EMF= 9V No currentflow. A rR E I rRIE IrIREMFE 1 9 9 )18( 9 )( )( )( VV RIV 881 VV RIV 111 EMF = 8V+1V 8 Volt 1 Volt EMF (6V) = 2V + 4V 4 Volt2 Volt Charges passing through wire Current flow Circuit complete Internal resistance Collision bet + ve ions with elec (drift velocity elec) - +
- 5. Eθ value DO NOT depend surface area of metal electrode. E cell = Energy per unit charge. (Joule)/C E cell- 10v = 10J energy released by 1C of charge = 100J energy released by 10C of charge Eθ – intensiveproperty–independentof amt – Ratio energy/charge Increasing surface area metal will NOT increase E cell Eθ Zn/Cu = 1.10V Surface area - 10 cm2 Total charge- 100C leave electrode E cell = 1.1V = 1.1 J energy for 1 C (charges leaving) 1C release 1.1 J energy 100 C release 110 J energy Voltmetermeasure energy for 1C – 110J/100C – 1.1V E cell no change Current– measured in Amp or Coulomb per s 1A = 1 Coulomb charge pass througha point in 1 s = 1C/s 1 Coulomb charge (elec) = 6.28 x 10 18 elec passing in 1 s 1 electron/protoncarry charge of – 1.6 x 10 -19 C ( very small) 6.28 x 10 18 electron carry charge of - 1 C ond electron ond Coulomb A sec.1 .1028.6 sec1 1 1 18 Surface area increase ↑ Total Energy increase ↑ Total Charge increase ↑Current increase ↑ BUT E cell remainSAME E cell = (Energy/charge) t Q I tIQ Q up ↑ – I up ↑ 100C flow 110J released VEcell Ecell eCh Energy Ecell 10.1 100 110 arg Surface area - 100 cm2 Total charge 1000C leave electrode E cell = 1.1V = 1.1 J energy for 1 C (charges leaving) 1 C release 1.1J energy 1000 C release 1100 J energy Voltmetermeasure energy for 1C – 1100J/1000C – 1.1V E cell no change VEcell Ecell eCh Energy Ecell 10.1 1000 1100 arg Eθ Zn/Cu = 1.10V 1000C flow 1100J released t Q I t Q I Surface area exposed 10 cm2 Surface area exposed 100 cm2
- 6. Relationship bet ∆G and Kc cellnFEG Relationship bet Energetics and Equilibrium cKRTG ln STHG Enthalpy change Entropy change Equilibrium constant Gibbs free energy change H G Relationshipbet ∆G, Kc and E cell cellnFEG STHG cKRTG ln cK Relationship bet Energetics and Cell Potential G cellE Gibbs free energy change Cell potential F = Faraday constant (96 500 Cmol-1) n = number electron Relationship bet ∆G, Kc and Ecell ΔGθ Kc Eθ/V Extent of rxn > 0 < 1 < 0 No Reaction Non spontaneous ΔGθ = 0 Kc = 1 0 Equilibrium Mix reactant/product < 0 > 1 > 0 Reaction complete Spontaneous ΔGθ Kc Eq mixture ΔGθ = + 200 9 x 10-36 Reactants ΔGθ = + 10 2 x 1-2 Mixture ΔGθ = 0 Kc = 1 Equilibrium ΔGθ = - 10 5 x 101 Mixture ΔGθ = - 200 1 x 1035 Products shift to left (reactant) shift to right (products) cellE G cK K nF RT E cell ln ΔGθ ln K Kc Eq mixture ΔGθ -ve < 0 Positive ( + ) Kc > 1 Product (Right) ΔGθ +ve > 0 Negative ( - ) Kc < 1 Reactant (left) ΔGθ = 0 0 Kc = 1 Equilibrium
- 7. E cell/Voltage– dependon natureof material Q nF RT EE ln T = Temp in K Q = Rxn Quotient E0 = std (1M) n = # e transfer F = Faraday constant (96 500C mol -1 ) R = Gas constant (8.31) cKRTQRTG lnln KRTG KRTQRTG o c ln lnln When ratio conc, Q = 1, all in std conc = 1M Non std condition 01ln 1 RT Q Q nF RT EE ln QRTGG o ln Non std condition o nFEG nFEG QRTnFEnFE ln Nernst equation Work or Free energyto do work dependon quantitymaterial and surface area E cell depend Nature of electrode Type of metal used Conc of ion Temp of sol Eθ Q T Current/I depend Surface area of contact Salt bridge conc Size of cation/anion Resistance high ↑ – current low ↓E cell depend Surface area of contact Salt bridge conc Size of cation/anion cellnFEG Gibbs free energy change do do WORK n = number electron F = Faraday constant (96 500 Cmol-1) Cell potential Increasing surface area → increase charge Q and I current - Work increase Current– dependon quantityand surface area
- 8. Zn ↔ Zn2+ + 2e Eθ = +0.76 Cu2+ + 2e ↔ Cu Eθ = +0.34 Zn + Cu2+ → Zn 2+ + Cu Eθ = +1.10V Zn half cell (-ve) Oxidation Cu half cell (+ve) Reduction Anode Cathode Zn(s) | Zn2+ (aq) || Cu2+ (aq) | Cu (s) Anode Cathode Half Cell Half Cell (Oxidation) (Reduction) Salt Bridge Flow electrons Zn/Cu Cell - 1M std condition -e -e Eθ cell = Eθ (cathode) – Eθ (anode) Eθ cell = +0.34 – (-0.76) = +1.10V Zn 2+ + 2e ↔ Zn (anode) Eθ = -0.76V Cu2+ + 2e ↔ Cu (cathode) Eθ = +0.34V Eθ cell = Eθ (cathode) – Eθ (anode) Zn 2+ + 2e ↔ Zn Eθ = -0.76V Cu2+ + 2e ↔ Cu Eθ = +0.34V Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 H2O + e- ↔ 1/2H2 + OH- -0.83 Zn2+ + 2e- ↔ Zn - 0.76 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 +0.17 Cu2+ + 2e- ↔ Cu + 0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 + +1.10 V Eθ Zn/Cu = 1.10V Cu2+ - - - - Zn Cu + + + + cellnFEG E cell with ∆G F = Faraday constant (96 500 Cmol-1) n = number electron cellnFEG kJJG G 212212300 10.1965002 Std electrodepotential- std reduction potential STD CONDITION Zn/Cu half cellCell diagram Q nF RT EE ln Ratio conc, Q = 1, all in std conc = 1M, T = 298K VE E 10.1 1ln 965002 298314.8 10.1
- 9. Zn ↔ Zn2+ + 2e Eθ = +0.76 2Ag++2e ↔ 2Ag Eθ = +0.80 Zn + Ag+ → Zn 2+ + Ag Eθ = +1.56V Zn half cell (-ve) Oxidation Ag half cell (+ve) Reduction Anode Cathode Zn(s) | Zn2+ (aq) || Ag+ (aq) | Ag (s) Anode Cathode Half Cell Half Cell (Oxidation) (Reduction) Salt Bridge Flow electrons -e -e Eθ cell = Eθ (cathode) – Eθ (anode) Eθ cell = +0.80 – (-0.76) = +1.56V Zn 2+ + 2e ↔ Zn (anode) Eθ = -0.76V Ag + + e ↔ Ag(cathode) Eθ = +0.80V Eθ cell = Eθ (cathode) – Eθ (anode) Zn 2+ + 2e ↔ Zn Eθ = -0.76V Ag+ + e ↔ Ag Eθ = +0.80V Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 H2O + e- ↔ 1/2H2 + OH- -0.83 Zn2+ + 2e- ↔ Zn - 0.76 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 +0.17 Cu2+ + 2e- ↔ Cu +0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 Cu+ + e- ↔ Cu +0.52 1/2I2 + e- ↔ I- +0.54 Fe3+ + e- ↔ Fe2+ +0.77 Ag+ + e- ↔ Ag + 0.80 1/2Br2 + e- ↔ Br- +1.07 + +1.56 V Ag Eθ Zn/Ag = +1.56V Ag+ - - - - + + + + Zn E cell with ∆G cellnFEG n = number electron F = Faraday constant (96 500 Cmol-1) cellnFEG kJJG G 301301000 56.1965002 Cell diagram Zn/Ag half cells Ratio conc, Q = 1, all in std conc = 1M, T = 298K Zn/Ag Cell - 1M std condition Q nF RT EE ln VE E 56.1 1ln 965002 298314.8 56.1 STD CONDITION
- 10. Zn half cell (-ve) Oxidation Cu half cell (+ve) Reduction Zn/Cu Cell -e -e Zn 2+ + 2e ↔ Zn Eθ = -0.76V Cu2+ + 2e ↔ Cu Eθ = +0.34V Zn ↔ Zn2+ + 2e Eθ = +0.76V Cu2+ + 2e ↔ Cu Eθ = +0.34V Zn + Cu2+ → Zn 2+ + Cu Eθ = +1.10V Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 H2O + e- ↔ 1/2H2 + OH- -0.83 Zn2+ + 2e- ↔ Zn - 0.76 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu + 0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 Cu+ + e- ↔ Cu +0.52 1/2I2 + e- ↔ I- +0.54 +1.10 V Cu2+ - - - - Zn Cu + + + + Q nF RT EE ln 1M 0.1M Zn2+ 10 ]1.0[ ]1[ ][ ][ 2 2 c c Q M M Cu Zn Q 0.1 M 1 M Using Nernst Eqn E0 = Std condition (1M) – 1.10V R = Gas constant (8.31) n = # e transfer(2 e) F = Faraday constant (96500C mol -1 ) VE E E 07.1 03.010.1 )10ln( )965002( )29831.8( 10.1 Non std 0.1M E cell decrease ↓ [Cu2+] decrease ↓ ↓ Le Chatelier’s principle Cu2+ + 2e ↔ Cu ↓ [Cu2+] decrease ↓ ↓ Shift to left ← ↓ E cell → less ↓ → Cu2+ less able ↓ to receive e- [Cu2+] ↓ E cell < Eθ 1.07 < 1.10 Zn/Cu half cellZn +Cu2+→Zn2++Cu NON STD CONDITION
- 11. Zn half cell (-ve) Oxidation Cu half cell (+ve) Reduction Zn/Cu Cell -e -e Zn 2+ + 2e ↔ Zn Eθ = -0.76V Cu2+ + 2e ↔ Cu Eθ = +0.34V Zn ↔ Zn2+ + 2e Eθ = +0.76V Cu2+ + 2e ↔ Cu Eθ = +0.34V Zn + Cu2+ → Zn 2+ + Cu Eθ = +1.10V Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 H2O + e- ↔ 1/2H2 + OH- -0.83 Zn2+ + 2e- ↔ Zn - 0.76 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu + 0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 Cu+ + e- ↔ Cu +0.52 1/2I2 + e- ↔ I- +0.54 +1.10 V Cu2+ - - - - Zn Cu + + + + Q nF RT EE ln 1M 10M Zn2+ 1.0 ]10[ ]1[ ][ ][ 2 2 c c Q M M Cu Zn Q 10 M 1 M Using Nernst Eqn E0 =Std condition (1M) – 1.10V R = Gas constant (8.31) n = # e transfer(2 e) F = Faraday constant (96500C mol -1 ) VE E E 13.1 03.010.1 )1.0ln( )965002( )29831.8( 10.1 Non std 0.1M E cell increase ↑ [Cu2+] increase ↑ ↓ Le Chatelier’s principle Cu2+ + 2e ↔ Cu ↓ [Cu2+] increase ↑ ↓ Shift to right → ↓ E cell → more ↑→ Cu2+ more able receive e- [Cu2+] ↑ E cell > Eθ 1.13 > 1.10 Zn/Cu half cellZn +Cu2+→Zn2++Cu NON STD CONDITION
- 12. Zn half cell (-ve) Oxidation Cu half cell (+ve) Reduction Zn/Cu Cell -e -e Zn 2+ + 2e ↔ Zn Eθ = -0.76V Cu2+ + 2e ↔ Cu Eθ = +0.34V Zn ↔ Zn2+ + 2e Eθ = +0.76V Cu2+ + 2e ↔ Cu Eθ = +0.34V Zn + Cu2+ → Zn 2+ + Cu Eθ = +1.10V Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 H2O + e- ↔ 1/2H2 + OH- -0.83 Zn2+ + 2e- ↔ Zn - 0.76 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu + 0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 Cu+ + e- ↔ Cu +0.52 1/2I2 + e- ↔ I- +0.54 +1.10 V Cu2+ - - - - Zn Cu + + + + Q nF RT EE ln 0.1M 1M Zn2+ 1.0 ]1[ ]1.0[ ][ ][ 2 2 c c Q M M Cu Zn Q 1 M 0.1 M Using Nernst Eqn E0 = Std condition (1M) – 1.10V R = Gas constant (8.31) n = # e transfer(2 e) F = Faraday constant (96500C mol -1 ) VE E E 13.1 03.010.1 )1.0ln( )965002( )29831.8( 10.1 Non std 0.1M E cell increase ↑ [Zn2+] decrease ↓ ↓ Le Chatelier’s principle Zn2+ + 2e ↔ Zn ↓ [Zn2+] decrease ↓ ↓ Shift to left ← ↓ E cell → more ↑→ Zn more able lose elec [Zn2+] ↓ E cell > Eθ 1.13 > 1.10 Zn/Cu half cellZn + Cu2+→ Zn2+ + Cu NON STD CONDITION
- 13. Cu half cell (-ve) Oxidation Cu half cell (+ve) Reduction -e Cu ↔ Cu 2+ + 2e Eθ = - 0.34V Cu2+ + 2e ↔ Cu Eθ = +0.34V Cu ↔ Cu2+ + 2e Eθ = - 0.34V Cu2+ + 2e ↔ Cu Eθ = +0.34V Cu + Cu2+ → Cu2+ + Cu Eθ = 0V Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 H2O + e- ↔ 1/2H2 + OH- -0.83 Zn2+ + 2e- ↔ Zn -0.76 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu + 0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 Cu2+ Zn Cu + + + + Q nF RT EE ln 0.1M 01.0 ]1.0[ ]001.0[ ][ ][ 2 2 c cathode anode c Q Cu Cu Q 0.1 M 0.001 M Using Nernst Eqn E0 = Std condition (1M) – 1.10V R = Gas constant (8.31) n = # e transfer(2 e) F = Faraday constant (96500C mol -1 ) VE E E 0285.0 0285.00 )01.0ln( )965002( )29831.8( 0 Cu2+/Cu half cell Cu + Cu2+ → Cu2+ + Cu -e Cu2+ 0.001M Cu (s) │Cu2+ (aq) (0.001M) ║ Cu2+ (aq) (0.1M)│Cu(s) - - - - Concentration cell Electrode same - diff conc Oxi cell – anode – lower conc Red cell – cathode – higher conc cathode anode Cu Conc cell made of Zn/Zn2+ Conc Zn2+- 0.11M and 0.22M. Find voltage. Zn (s) │Zn2+ (aq) (0.11M) ║ Zn2+ (aq) (0.22M)│Zn(s) Zn + Zn2+ → Zn2+ + Zn cathode anode 0.22M 0.11 M 5.0 ]22.0[ ]11.0[ ][ ][ 2 2 c cathode anode c Q Zn Zn Q Q nF RT EE ln VE E 0089.0 )5.0ln( )965002( )29831.8( 0
- 14. Fe half cell (-ve) Oxidation Fe half cell (+ve) Reduction -e Fe ↔ Fe 2+ + 2e Eθ = + 0.45V Fe2+ + 2e ↔ Fe Eθ = - 0.45V Fe ↔ Fe2+ + 2e Eθ = + 0.45V Fe2+ + 2e ↔ Fe Eθ = - 0.45 V Fe + Fe2+ → Fe2+ +Fe Eθ = 0V Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 H2O + e- ↔ 1/2H2 + OH- -0.83 Zn2+ + 2e- ↔ Zn -0.76 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Fe2+ Zn Fe + + + + Q nF RT EE ln 0.1M 1.0 ]1.0[ ]01.0[ ][ ][ 2 2 c cathode anode c Q Fe Fe Q 0.1 M 0.01 M Using Nernst Eqn E0 = Std condition (1M) – 1.10V R = Gas constant (8.31) n = # e transfer(2 e) F = Faraday constant (96500C mol -1 ) VE E E 029.0 029.00 )1.0ln( )965002( )29831.8( 0 Fe2+/Fe half cell Fe + Fe2+ → Fe2++ Fe -e Fe2+ 0.01M Fe(s)│Fe2+ (aq) (0.01M) ║ Fe2+ (aq) (0.1M)│Fe(s) - - - - Concentration cell Electrode same - in diff conc Oxi cell – anode – lower conc Red cell – cathode – higher conc cathode anode Fe Find cell potential Mn (s) │Mn2+ (aq) (0.1M) ║ Pb2+ (aq) (0.0001M)│Pb(s) Mn + Pb2+ → Mn2+ + Pb 0.0001M 0.1 M cathode anode 001.0 ]0001.0[ ]1.0[ ][ ][ 2 2 c cathode anode c Q Pb Mn Q Q nF RT EE ln VE E 96.0 )001.0ln( )965002( )29831.8( 05.1
