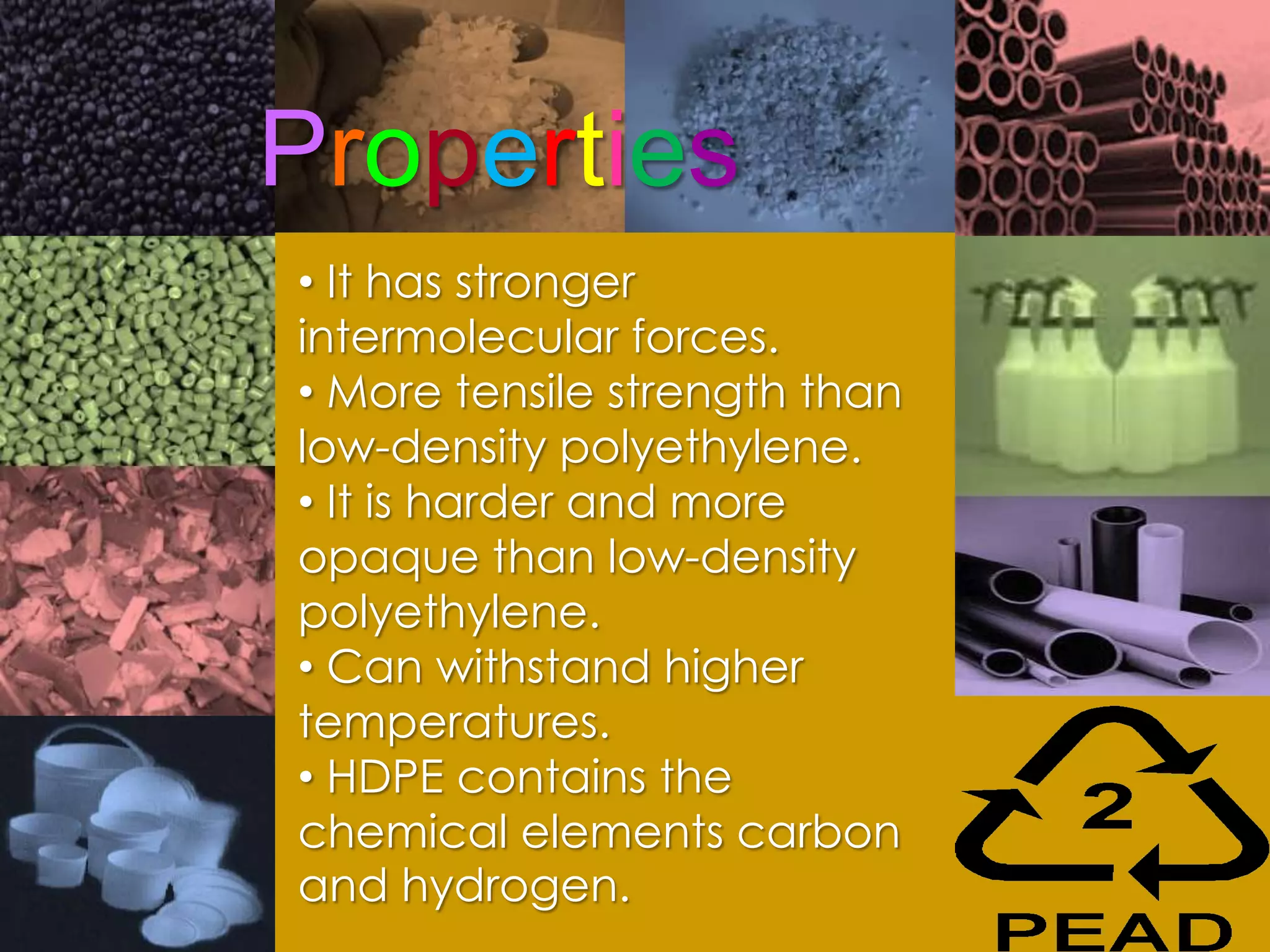
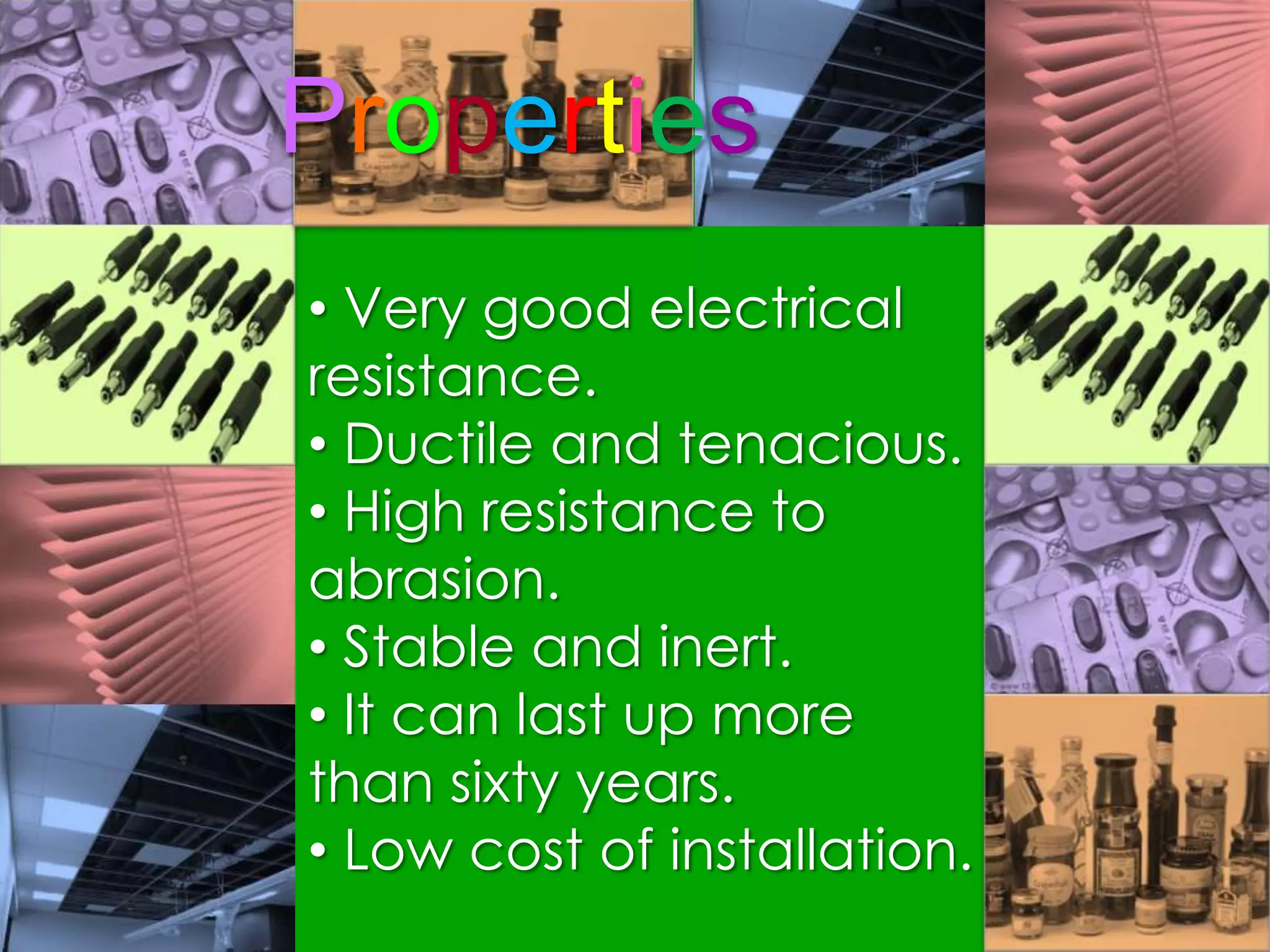
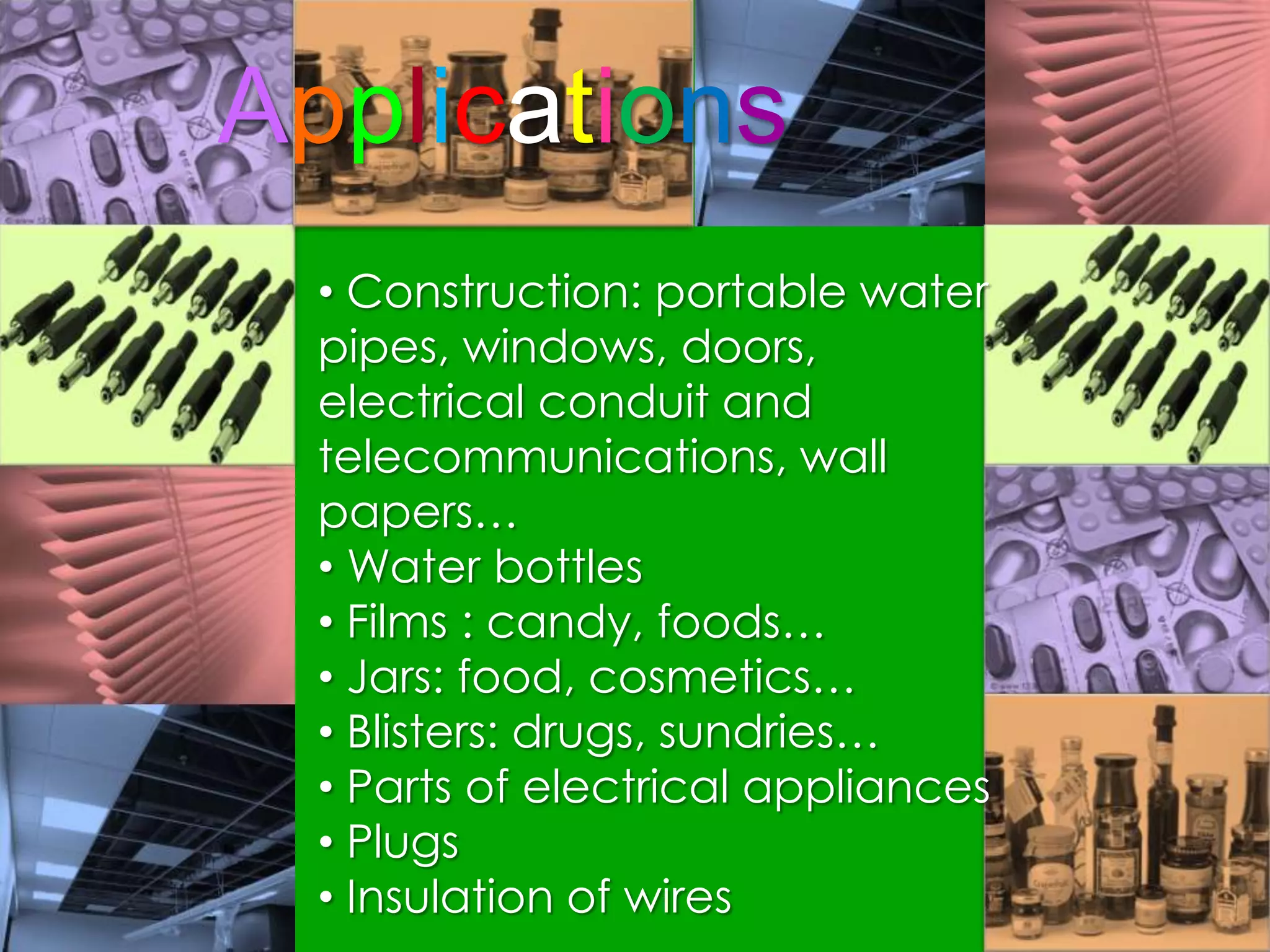
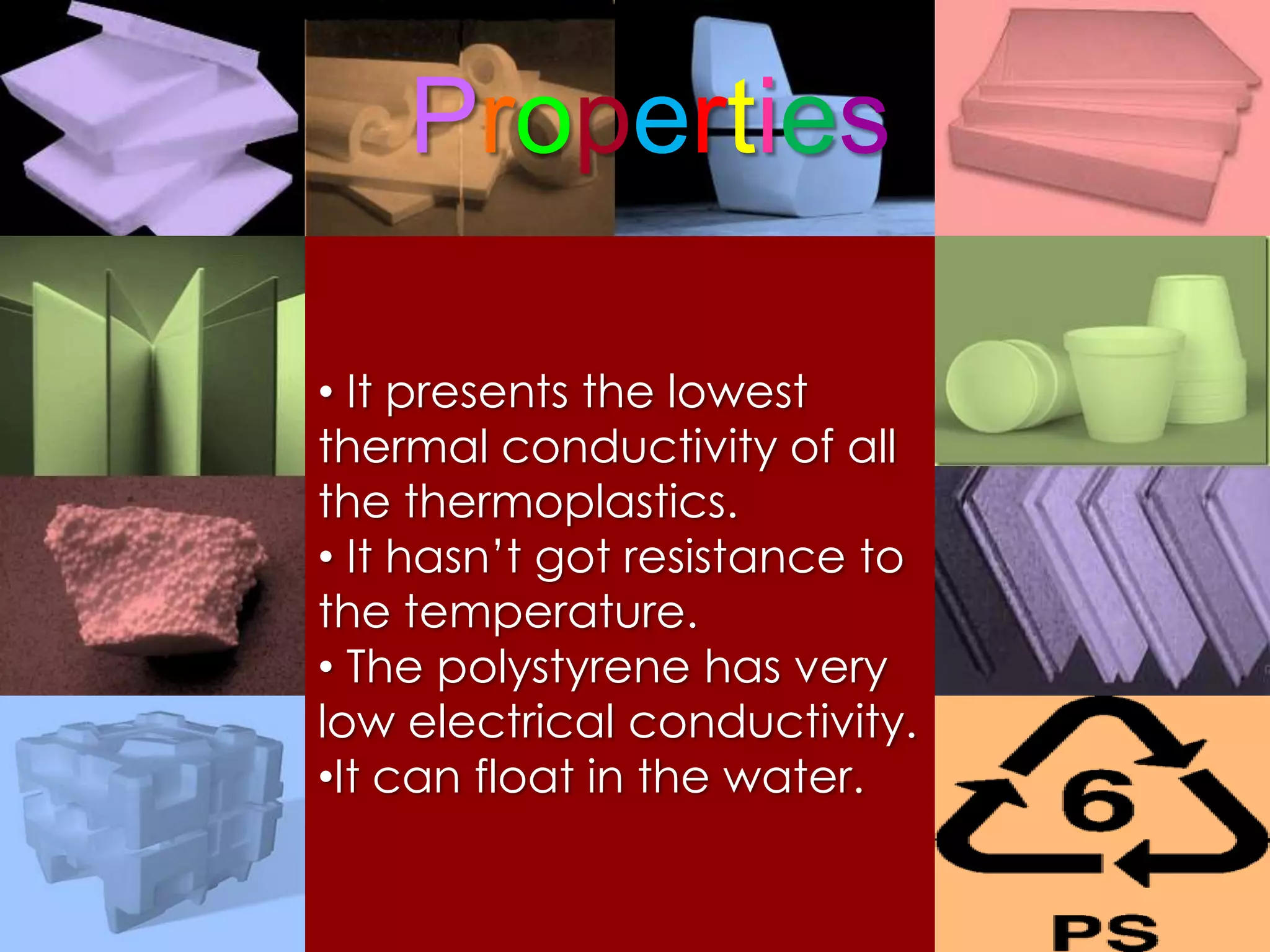
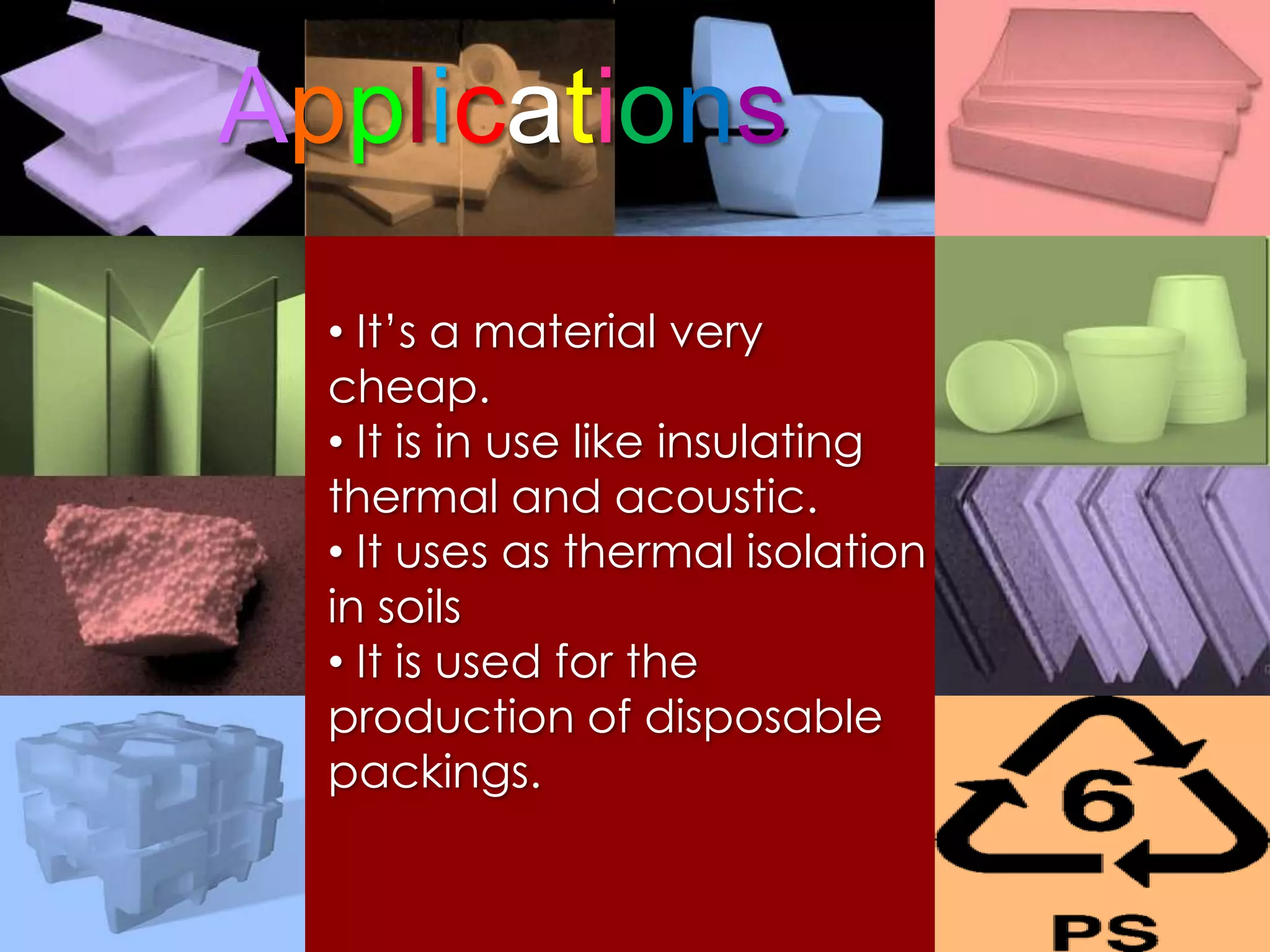
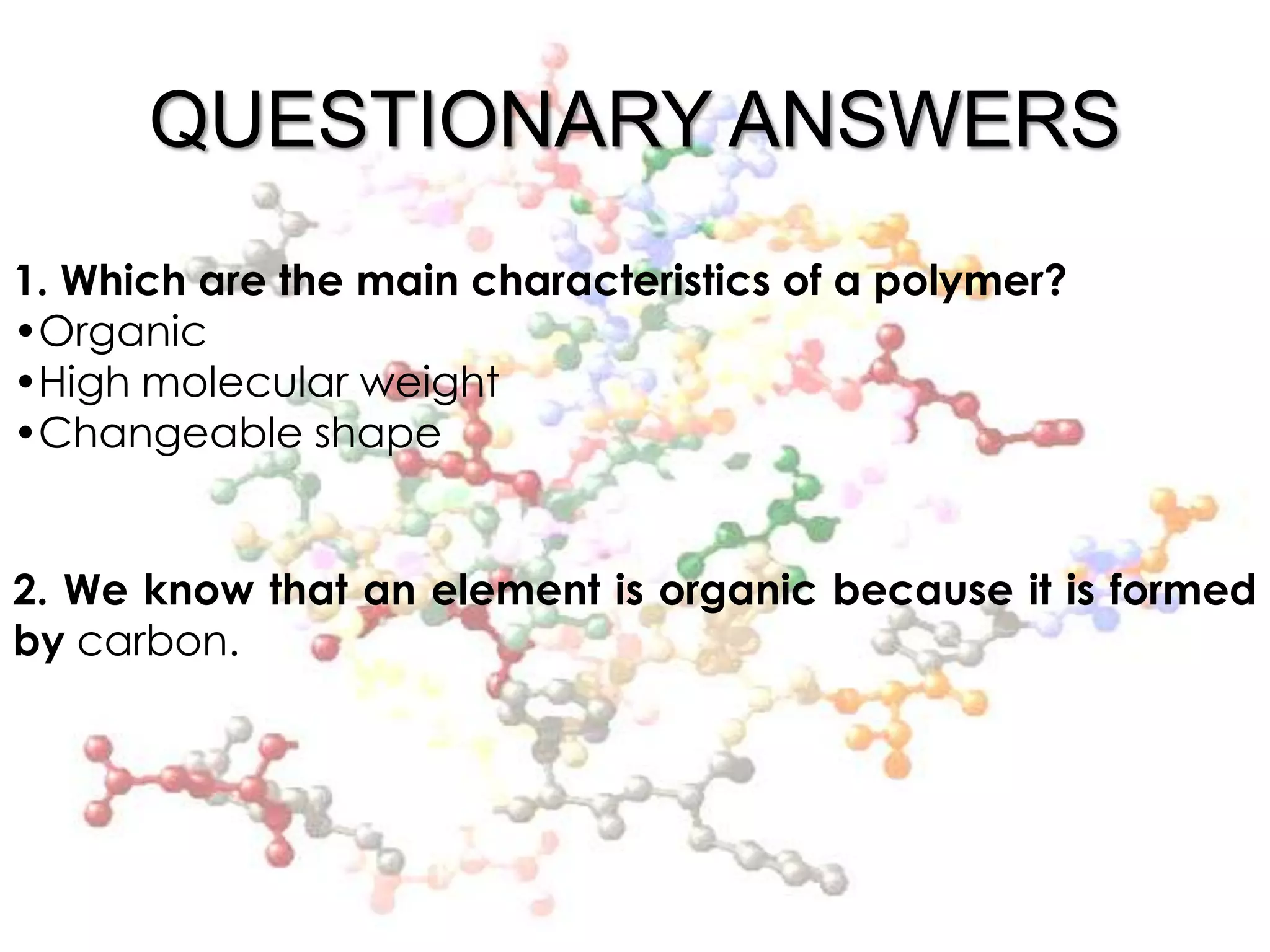
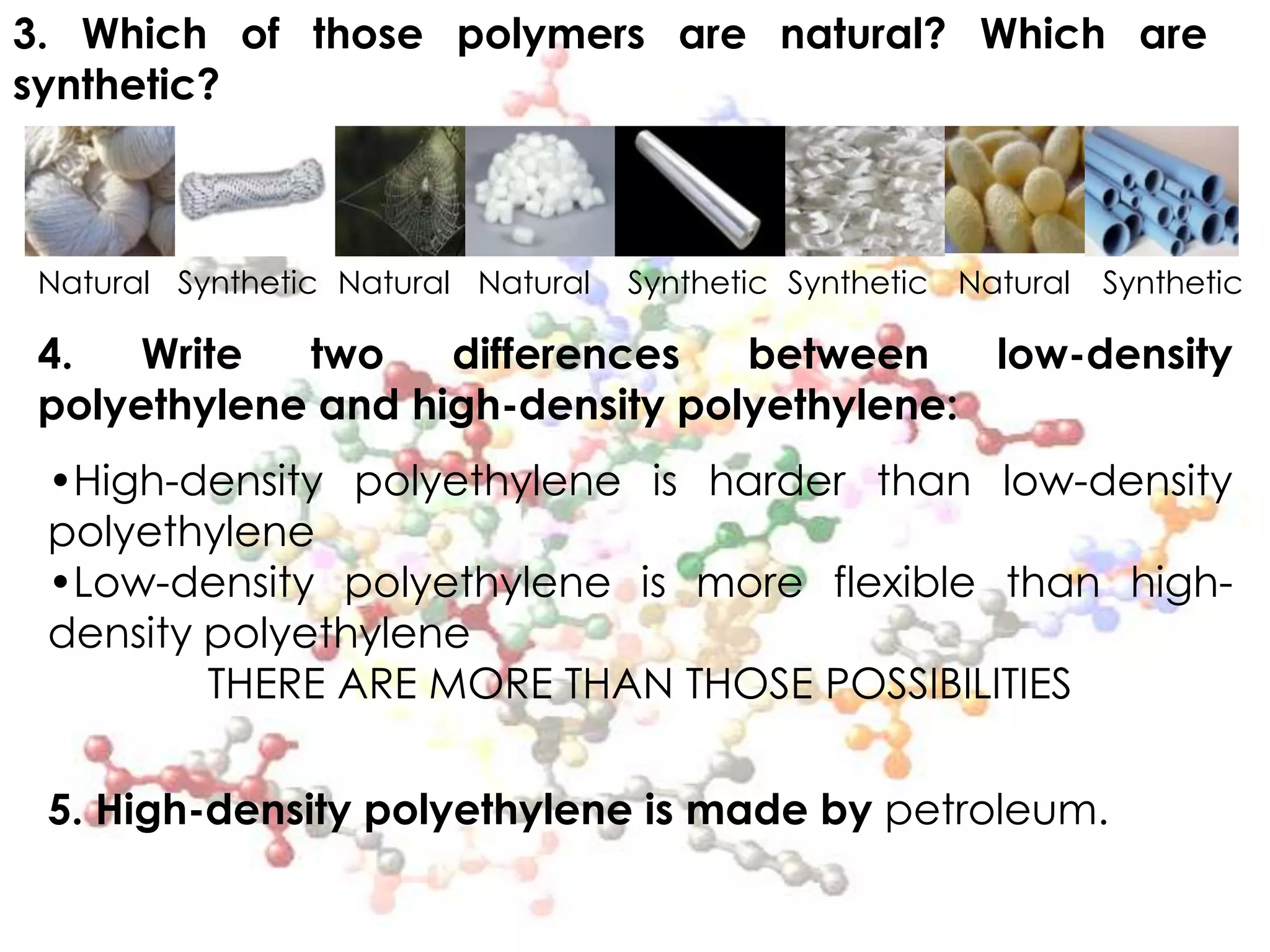
Polymers are organic macromolecules formed by the union of monomers through polymerization. There are natural polymers like polyethylene and synthetic polymers like polyvinyl chloride. The document discusses several common polymers - low-density polyethylene, high-density polyethylene, polypropylene, polyethylene terephthalate, polyvinyl chloride, and polystyrene - outlining their properties and applications. Key uses include plastic bags, bottles, films, construction materials, and more. The document also includes questions and answers about polymer characteristics and specific polymers.