Energy Of Reaction
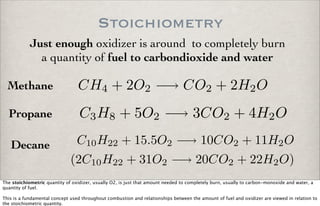
- 1. Stoichiometry Just enough oxidizer is around to completely burn a quantity of fuel to carbondioxide and water CH4 + 2O2 −→ CO2 + 2H2O C3H8 + 5O2 −→ 3CO2 + 4H2O C10H22 + 15.5O2 −→ 10CO2 + 11H2O (2C10H22 + 31O2 −→ 20CO2 + 22H2O) Methane Propane Decane The stoichiometric quantity of oxidizer, usually O2, is just that amount needed to completely burn, usually to carbon-monoxide and water, a quantity of fuel. This is a fundamental concept used throughout combustion and relationships between the amount of fuel and oxidizer are viewed in relation to the stoichiometric quantity.
- 2. Stoichiometry CH3OH + 1.5O2 −→ CO2 + 2H2O Methanol Methyl Butanoate (simple ester used to model ester biofuels) CH3CO2C3H7 + 6.5O2 −→ 5CO2 + 5H2O (2CH3CO2C3H7 + 13O2 −→ 10CO2 + 10H2O The same even with oxygenated fuels The same holds for even oxygenated compounds. In this case the oxygen comes not only from O2, but also from the fuel itself. But, nevertheless, in the stoichiometric mixture, the atom amounts balance.
- 3. Balancing Hydrocarbon Equations CnHmOp + xO2 −→ yCO2 + zH2O •All the carbons atoms go to CO2 •All the hydrogens go to H2O •Count the number of oxygens on the right hand side •Subtract the number of oxygens in the fuel. Balancing a chemical equation uses the fundamental fact that during a chemical reaction the number of atoms on each side of the equation stay the same. In balancing a combustion equation to CO2 and water, we have a special case which simplifies determining how much fuel, oxidizer and products are needed to balance the equation. Essentially one recognizes that all the carbon of the fuel goes to CO2, all the hydrogens go to H2O. Secondly, all the oxygens on the right hand side, the product side, come from the fuel and what is left over comes from pure O2.
- 4. 90 RON in air Balance the equation for the complete combustion of 90 RON mixture of heptane/isooctane in air: •90 RON mixture •10% heptane •90% isooctane •The oxidizer is air (approximately just nitrogen and oxygen): •79% Nitrogen •21% Oxygen (.90 C8H18 + . 0.1 C7H16) + x(0.79 N2 + 0.21 O2) −→ y CO2 + z H2O + 0.79x N2 A typical problem is to find the stoichiometric quantities needed of a mixtures of hydrocarbons in air. In this case, we are not dealing with simple integer numbers for the quantities in the balanced equations. But, nevertheless, the steps to compute the balanced equation are the same. The quantities x,y,z are just not integers.
- 5. 90 RON in Air •How many Carbons? •Left Hand Side •Carbons from Heptane + Carbons from isooctane •Each multiplied by percentage due to RON •(0.10)(7) + (0.90)(8) = 7.9 •Right Hand Side •This is the number of CO2 molecules •7.9 To actually balance the equation of our example, we start with computing the number of carbons. On the left hand side, the source of carbons is the 90 RON primary reference fuel, meaning a mixture of 10% heptane and 90% isooctane. This means that there is an e!ect number of carbons of 7.9 (notice it is between 7 and 8 carbons). This has to balance with the right hand side. But the only carbons on this side is the one carbon of CO2, so that means there are 7.9 CO2 molecules produced.
- 6. 90 RON in Air •How many hydrogens? •Left hand side •(0.1) hydrogens in heptane + (0.90) hydrogens in isooctane •(0.10)(16) + (0.90)(18) = 17.8 •Right hand side •hydrogens in water •17.8/2 = 8.9 waters How many hydrogens are there? Using the same logic, 10% of heptane and 90% of isooctane, means that 10% of the 16 hydrogens come from heptane and 90% of the 18 hydrogens come from isooctane. This means there are e!ectively 17.8 hydrogens in the fuel (once again notice that it is something between 17 and 18 hydrogens). All the hydrogens on the right hand side are in water. There are two hydrogens per water, so there are 8.9 waters.
- 7. RON 90 in AIR •How many oxygens on the left hand side? •All in the air (no oxygens in the fuel) •Right hand side •oxygens in carbonmonoxide + oxygens in water •(7.9)(2) + (8.9)(1) = 24.7 •Left hand side •oxygens in air = oxygens in right hand side •x (2)(0.21) = 24.7 •solving: x=58.8 Now we have to balance the oxygens whose only source in the reactants is in air. Starting with the right hand side (since now the molecule amounts are not fixed) we add up the oxygens in both carbonmonoxide and water. This gives a total of 24.7 oxygens. This means on the left hand side, that the oxygens in air have to total 24.7. Since oxygen molecules are only 21% of the mixture and there are two oxygens per oxygen molecule, x times 2 times 0.21 has to equal 24.7. This means that there are 58.8 portions of air.
- 8. 90 RON in Air Final Balanced Equation (.90 C8H18 + . 0.1 C7H16) + 58.8(0.79 N2 + 0.21 O2) −→ 7.9 CO2 + 8.9 H2O + 46.5 N2 So the final balanced equation looks like this.
- 9. Fuel-Air Ratio by Weight From the previous slide, the fuel air ratio is: 1 mole fuel per 58.8 moles air However, usually, a fuel air mixture is done by weight. x grams of fuel per y grams of air. Another quantity is the percent, by weight, of fuel needed. This is easily computed from the air-fuel ratio. From the balanced example, we saw that there was 1 mole of 90 RON fuel to 58.8 moles of air. Since in the laboratory, the number of moles cannot be directly measured, it is often more convenient to use units that are directly measurable, such as weight and pressure. More often than not, in engineering and combustion papers and tables, fuel and air quantities are given in kilograms and pressure. Fortunately, this is a simple conversion of units. Fuel to air ratio, by weight is also a useful measure, representing a measurable percentage.
- 10. Air Fuel Ratio •Molecule Weight •Heptane: 100.2 g/mol •Isooctane: 114.23 g/mol •90 RON Fuel •(0.1)(heptane) + (0.90)(isooctane) •(0.10)(100.2) + (0.90)(114.23) = 112.8 g/mol •Air •(0.79)(Nitrogen) + (0.21)(oxygen) •(0.79)(28) + (0.21)(32.0) = 28.8 g/mol To convert from moles to grams, of course we need the molecular weight of the components of the fuel, namely heptane and isooctane. To compute the e!ective molecular weight of the 90 RON fuel, we take 10% of the heptane and 90% of the isooctane molecular weight, giving 112.8 g/mol. The same principle is used to compute the e!ective molecular weight of air, 79% nitrogen and 21% oxygen, giving 28.8 g/mol.
- 11. Air-Fuel Ratio •Air-Fuel Ratio • (58.8 mol air)(28.8 g/mol) = 1693 g •(1 mol fuel)(112.82 g/mol) = 112.8 g •(1693 g)/(112.8 g) = 15 •Percentage fuel by Weight •(1 g fuel)/15 g air) = 0.066 •Percent: 6.6% Using the parameters of the last example, we have 58.8 moles of air, which means we have 1693 grams of air, with 1 mole of fuel, giving 112.8 grams of fuel. This yields an air to fuel ratio of 15. Another quantity used is percentage weight of the fuel. This is simply the inverse of the air-fuel ratio multiplied by 100.
- 12. Examples Fuel By Weight By Volume Percent (weight) Gasoline 14.7:1 - 6.8 Natural Gas 17.2 9.7:1 5.8 Propane (LP) 15.5:1 23.9:1 6.45 Ethanol 9:1 - 11.1 Methanol 6.4:1 - 15.6 Hydrogen 34:1 2.39:1 2.9 Diesel 14.6:1 - 6.8 Here is a table from the literature of the di!erent typical ratios for common fuels, gasoline, natural gas, propane, ethanol, methanol, hydrogen and diesel.
- 13. Ethalpy dh = ( δh δT )P dT + ( δh δP )T dP dh = ( δh δT )P dT ∆H = HS0 + ! S1 S0 CP dT For a constant pressure reaction: Integrating both sides from state 0 and state 1: Under constant pressure, the total di!erential of h(T,P) the dP term is zero. If the result is integrated between state 1 and state 2, using the expression for Cp, then the expression for the change in enthalpy between state 1 and state 2 is derived.
- 14. Heat of Formation Definition: Enthalpy (Heat) of Formation The reference state of the elemental structures at Room Temperature and Pressure (298 Kelvin, 1 atm) The following have zero enthalpy of formation: H2, O2, C(graphite), N2 As a consequence of the first law of thermodynamics, we do not have to deal with absolute energy values such as the total energy of a molecule, but relative energy values. In addition, since the first law says we only have to deal with the ‘before’ and ‘after’ states, we can pick an arbitrarily convenient reference state and compare all values relative to it and even not worry about how we got there. In fact, whether the actual process goes through this state is irrelevant. Heat of formation uses the individual atoms as the base reference state at a standard temperature and pressure, 298 Kelvin and 1 atmosphere. These individual atoms are defined to have a heat of formation of zero. In computing the enthalpy change between state 1 and state 2, we will take a detour through this reference state.
- 15. Heat of Combustion !"#$%&'%(&)*+,$-&. (/0%1!2324567%%%%%%0%!0/%%1!0864937 1!:;04087 (!8%1!984:57%%%%%/0%1;7 !"#$%&'%<&=)#$-&. &'%$>"%?"#@$#.$, 1984:57 !"#$%&'%<&=)#$-&. &'%$>"%A=&B+@$, 1!:994;37 (1C=#D>-$"7%%%%%0%!0%%%%%%0%/0 Consequence of the first law of thermodynamics (a computable path is chosen) Once again, as a consequence of the first law of thermodynamics, we can use heats of formation to compute the heat of combustion. Essentially, we create a fictitious energy path. We start with the reactant molecules and break them apart into individual atoms. The energy needed to do this is the heat of formation. We then recombine these atoms into the product molecules, and, once again the energy needed is the heat of formation. Obviously, the actual path in going from reactants to products does not follow this path, but using the first law of thermodynamics, we can use are reference states for the computation.
- 16. Heating Value •Higher Heating Value •Thermodynamic heat of combustion •Enthalpy change for the reaction with the same temperature before and after combustion. •Lower Heating Value •Heat of Vaporization subtracted from higher heating value •water component is in a vapor state after combustion Heat released when a given amount of fuel is combusted. A standard measurement used in industry for the heat content of a species, meaning how much heat is released when the species is combusted. Two values are used. The first is higher heating value. This is the thermodynamic heat of combustion, meaning the enthalpy change of combustion at a constant temperature. The second is the lower heating value. This is where the heat of vaporization is subtracted from the higher heating value.
- 17. Heating Value Relationship hvapor,H2O(nH2O,out/nH2O,in) Higher Heating Value = Lower Heating Value + The lower heating value is useful for boilers where in the end the water is evaporators The di!erence between the higher and the lower heating value is basically related to the heat of vaporization of water. It is for this reason that the lower heating value is useful for boilers.
- 18. Lower Heating Value: Fuels Small hydrocarbons: 46 to 50 MJ/kg Higher hydrocarbons: around 50 MJ/kg Hydrogen: 120 MJ/kg Carbon Monoxide 10.112 MJ/kg Esters 30-40 MJ/kg Alcohols 18-30 MJ/kh This is a general comparison of the heating values of various groups of fuels. One notable fact is that the hydrocarbons have a much higher heating value than the typical biofuels, such as alcohol or esters (which are derived from, for example, rapseed oil). It is also noteworthy that hydrogen has the highest heating value.
- 19. Hydrocarbons Methane Ethane Propane Butane Pentane Hexane Heptane Octane Nonane Decane Undecane Dodecane 43 44 45 46 47 48 49 50 44.147 44.194 44.24 44.311 44.427 44.566 44.752 45.357 45.752 46.357 47.794 50.009 Lower Heating Value These are the lower heating values of a series of hydrocarbon fuels, increasing in number of carbons. Methane has the highest heating value among them.
- 20. Esters Methoxymethane Ethoxyethane Propoxypropane Butoxybutane 25 28 31 34 37 40 37.798 36.355 33.867 28.783 Lower Heating Value Among the esters, those fuels similar to rapseed (or other) oils for biofuels, the larger esters have a higher heating value.
- 21. Ethanols Methanol Ethanol n-propanol Isopropanol 18 20 22 24 26 28 30 32 30.447 30.68 28.865 19.937 Lower Heating Value Another class of biofuels are the ethanols. Other than methanol, these have a heating value of around 30 MJ/kg.
- 22. Temperature Dependence But no heat release Temperature Rise But the temperature stays constant Heat Lost to the Environment Enthalpy Temperature Constant Temperature Process Adiabatic Process Reactants Products Heat Loss And Temperature Gain The enthalpy of a species (or set of species) is a function of temperature. For example, the total enthalpy of a set of reactants is a function of temperature as is the total enthalpy of a set of products. This means that to find the gain or loss of energy to the system we need to know the beginning and ending temperatures. It can also be seen in this figure that, for example, an adiabatic process, meaning that there is no change in energy from reactants to products, there is an increase in temperature. The energy is not lost to the environment, but given to the products in raising their temperature. We will see in the next slides that this is defined as the adiabatic flame temperature.
- 23. Adiabatic Flame temperature Enthalpy Temperature Reactants Products Constant Enthalpy (Adiabatic) Adiabatic Flame Temperature Adiabatic Flame Temperature is important to combustion because it represents the highest possible temperature a given combustion process can achieve. It provides another tool to compare fuels. The term adiabatic is that no heat is lost to the environment, i.e. the enthalpy of the system stays constant. The energy produced by the reaction is put into raising the temperature of the products. Of course, no process is perfect and not all of the heat can be converted to temperature, so this represents the highest theoretical limit. But it gives a good idea of the potential of a fuel.
- 24. Calculation CH4 + 2(O2 + 3.76N2) −→ CO2 + H2O + 7.52N2 Complete Combustion of Methane Hreact = ! react Nihi,298 = Hproducts = ! prod Njhj,T Adiabatic Flame Temperature The adiabatic flame temperature is solved for by setting the heat of combustion of the reactants equal to the heat of combustion of the products. The term adiabatic means there is no loss of heat. This means that all the heat in the reactants goes into the products. For example, let us look at the complete combustion of methane in air.
- 25. Enthalpies Hreact,298 = hch4 + 2ho2 + hn2 Hprod,T (hco2,298 + CP,co2(T − 298)) (hh2o,298 + CP,h2o(T − 298)) (hn2,298 + CP,n2(T − 298)) = (assume constant heat capacity) match and solve for T The temperature of the heat of combustion of the reactants is know, namely the standard temperature of 298, so the standard enthalpy of each of reactants can be used. The enthalpy of the reactants is their sum. However, the temperature of the products, i.e. the adiabatic flame temperature, is not known and has to be solved for. The main problem is that the heat capacity is also a function of temperature. However, if we assume an average heat capacity, then the we have one linear equation and one unknown, T.
- 26. Examples hi,298 Cp,1200 CH4 CO2 H2O N2 O2 Species -74.831 --- -393.546 56.21 -241.845 43.87 0 33.71 0 ---- The standard heats of formation, one atm and 298K, for both the reactants and products can be looked up. Since the heat capacity changes with temperature, we make an assumption and choose the heat capacity at a temperature somewhere between 298 and the expected flame temperature. In this case we choose 1200K. We these values the flame temperature can be solved for.
- 27. Examples Fuel in air in oxygen Methane 1957 2810 Ethane 1960 Propane 1980 2020 Butane 1970 Hydrogen 2045 2660 Acetylene 2400 3100 Here are some examples of the adiabatic flame temperature both in air and in pure oxygen for the stoichiometric full complete combustion. The adiabatic flame temperature in air is always lower than that in pure oxygen because even though the stoichiometry between the fuel and oxygen is the same in both cases, in air not only do the combustion products have to be heated up but also the inert nitrogen.
- 28. Equivalence Ratio The adiabatic flame temperature peaks around equivalence ratio of 1.0. In one sense that is where the most e!cient stoichiometric combustion occurs. However, closer examination is that the peak is a bit to the rich side. The accepted explanation for this is disassoication e"ect. At higher temperatures, the equilibrium, particularly CO2 is shifted toward its disassociation products, for example CO. This is an example of the fact that incomplete combustion can always occur, we do not always have the ideal case.
