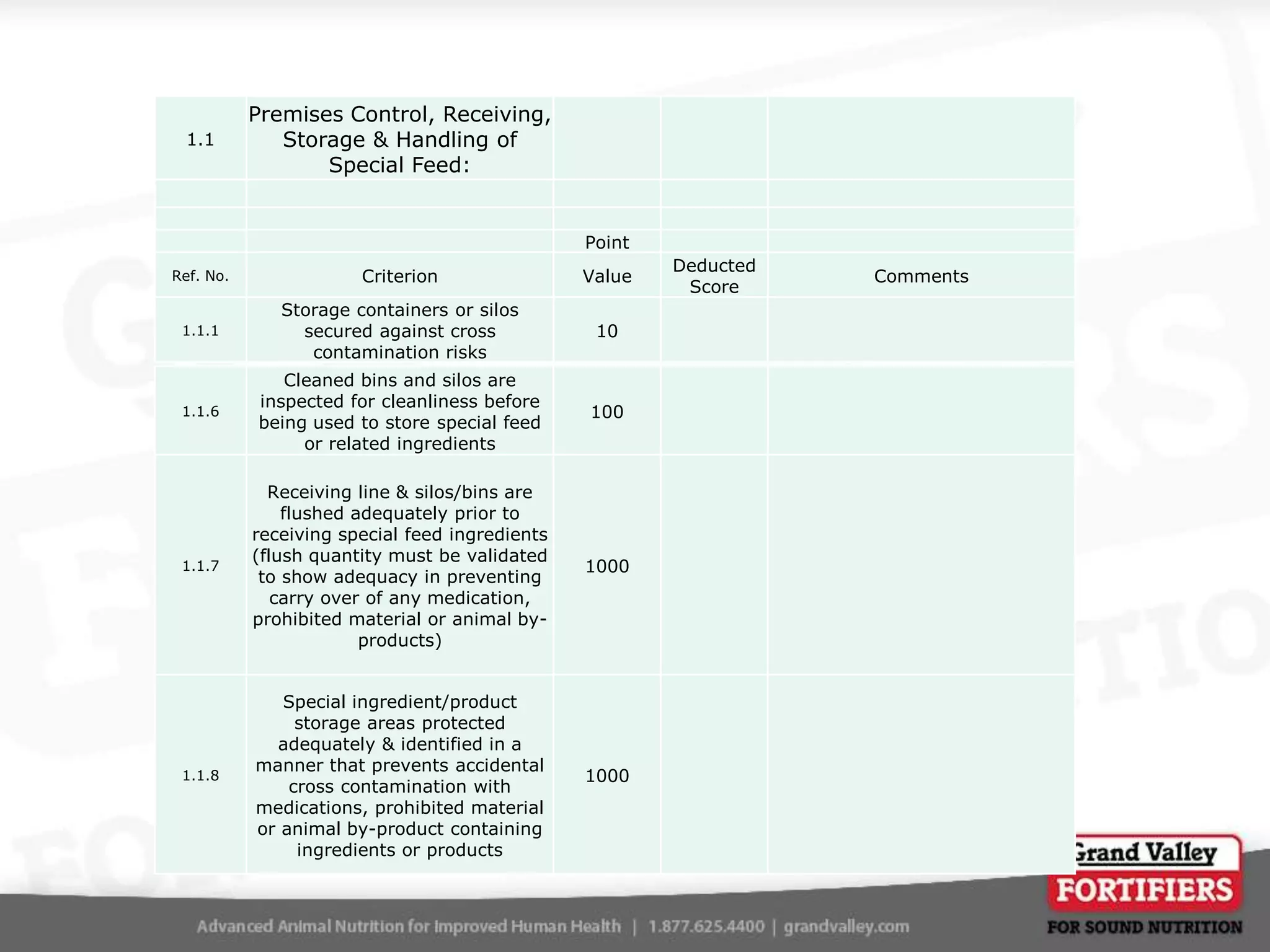
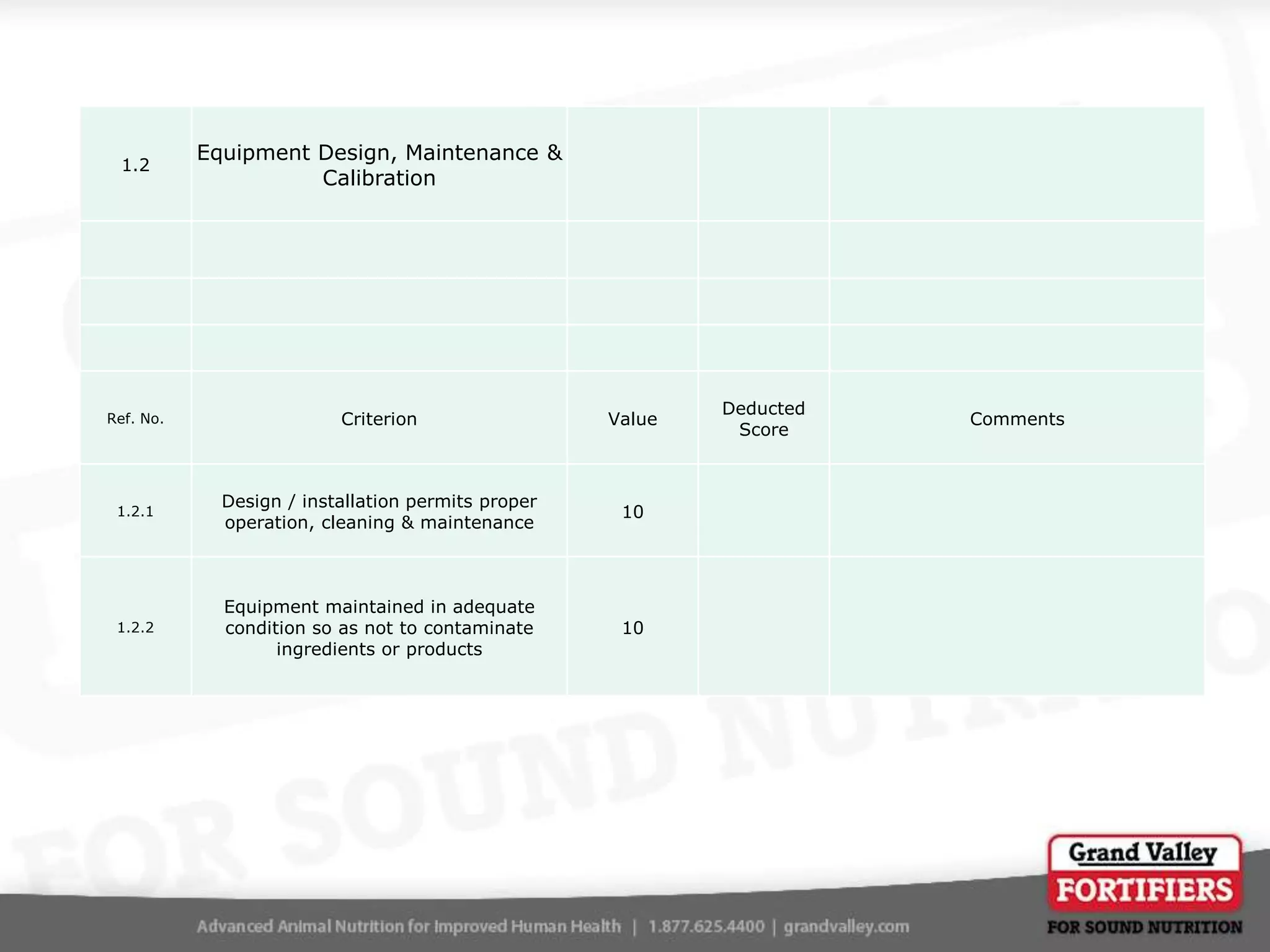
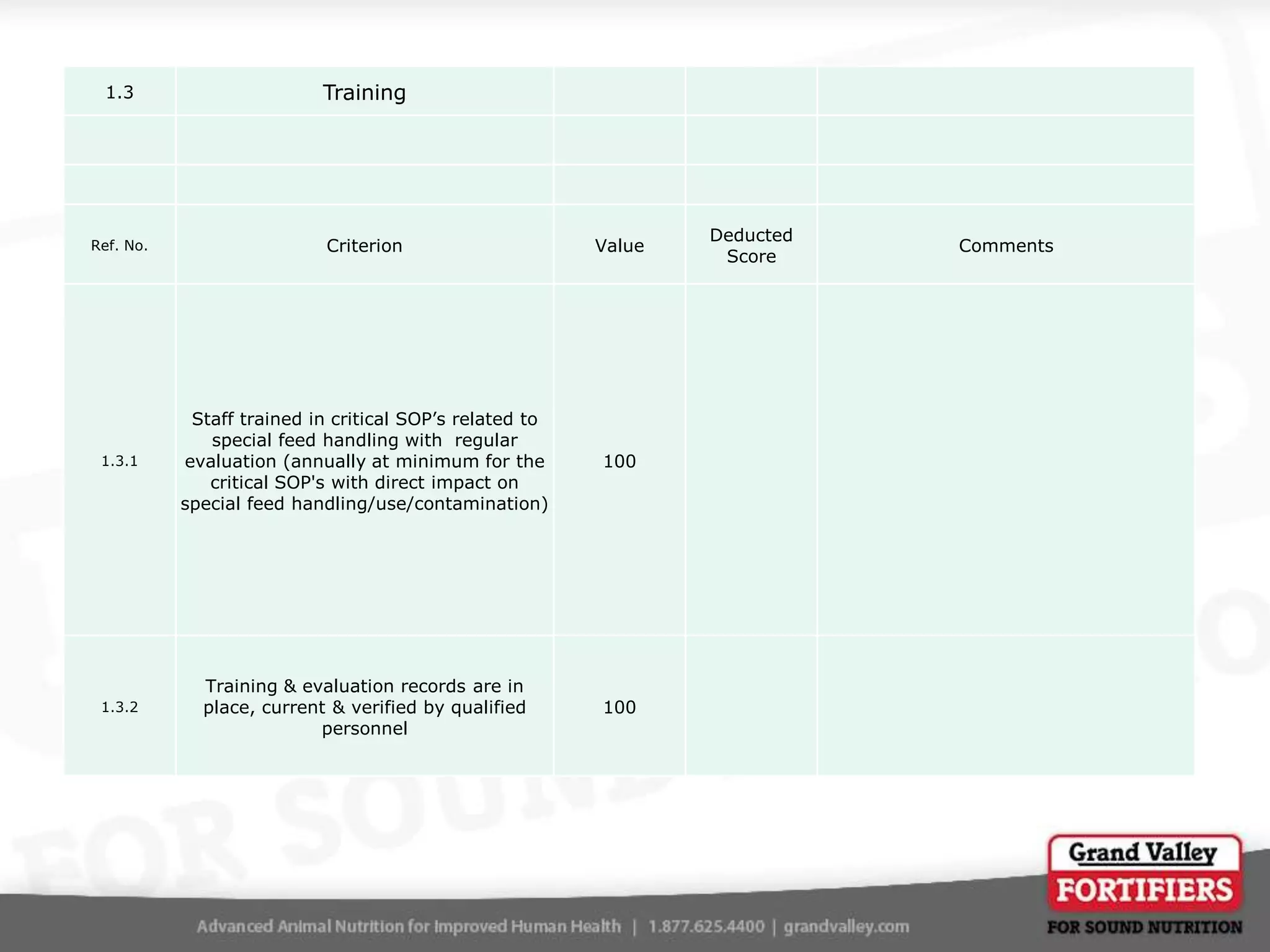
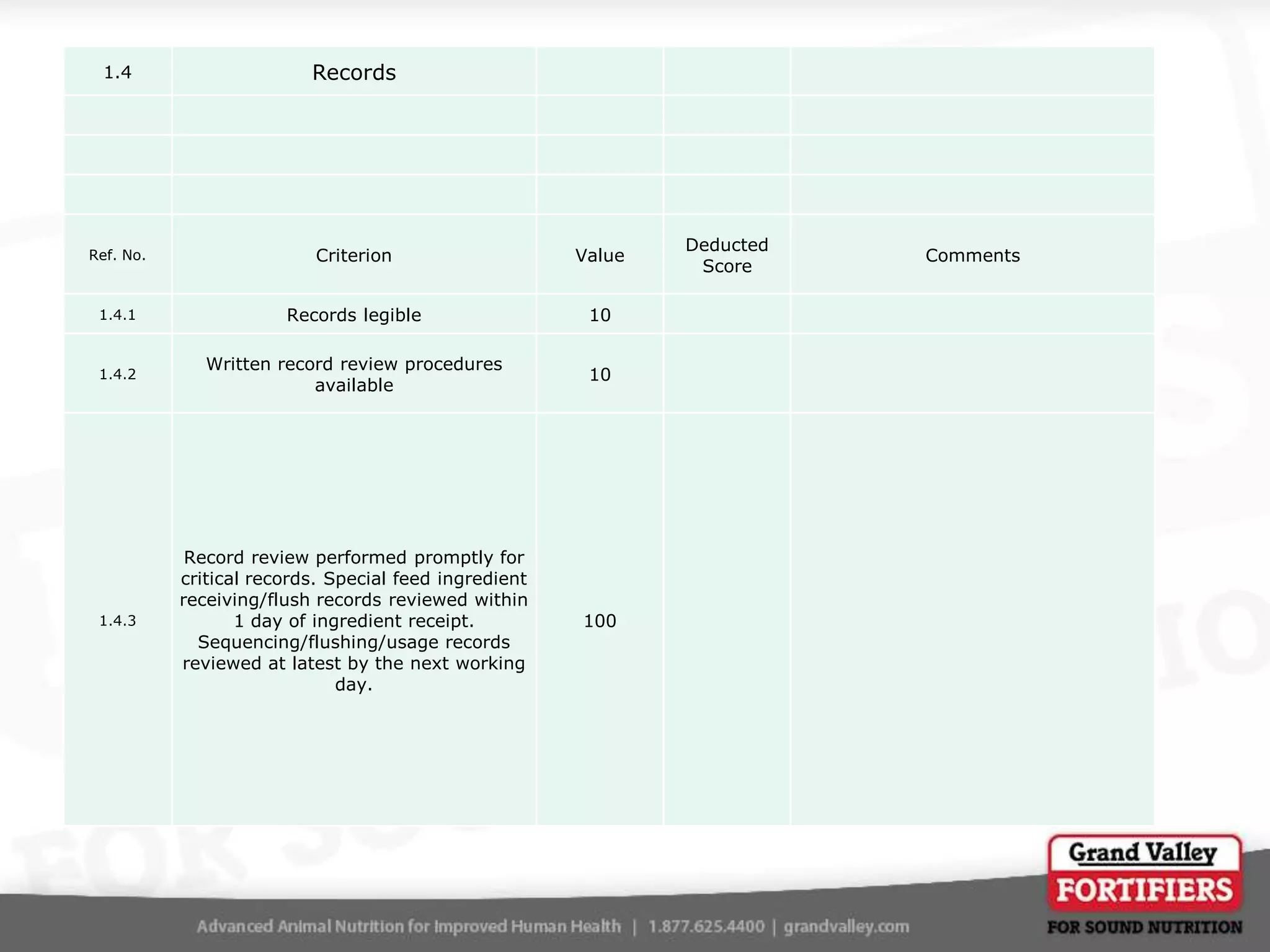
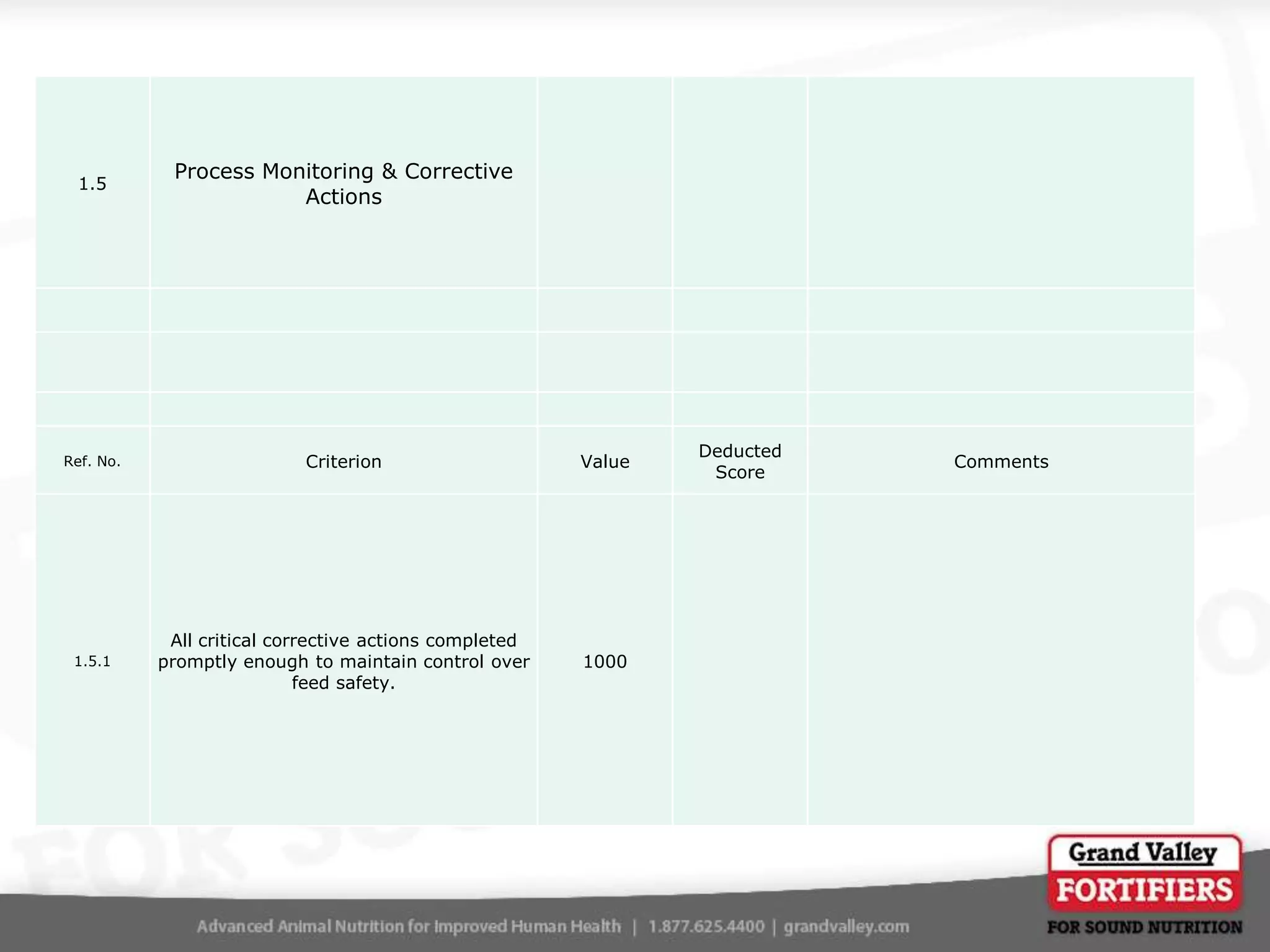
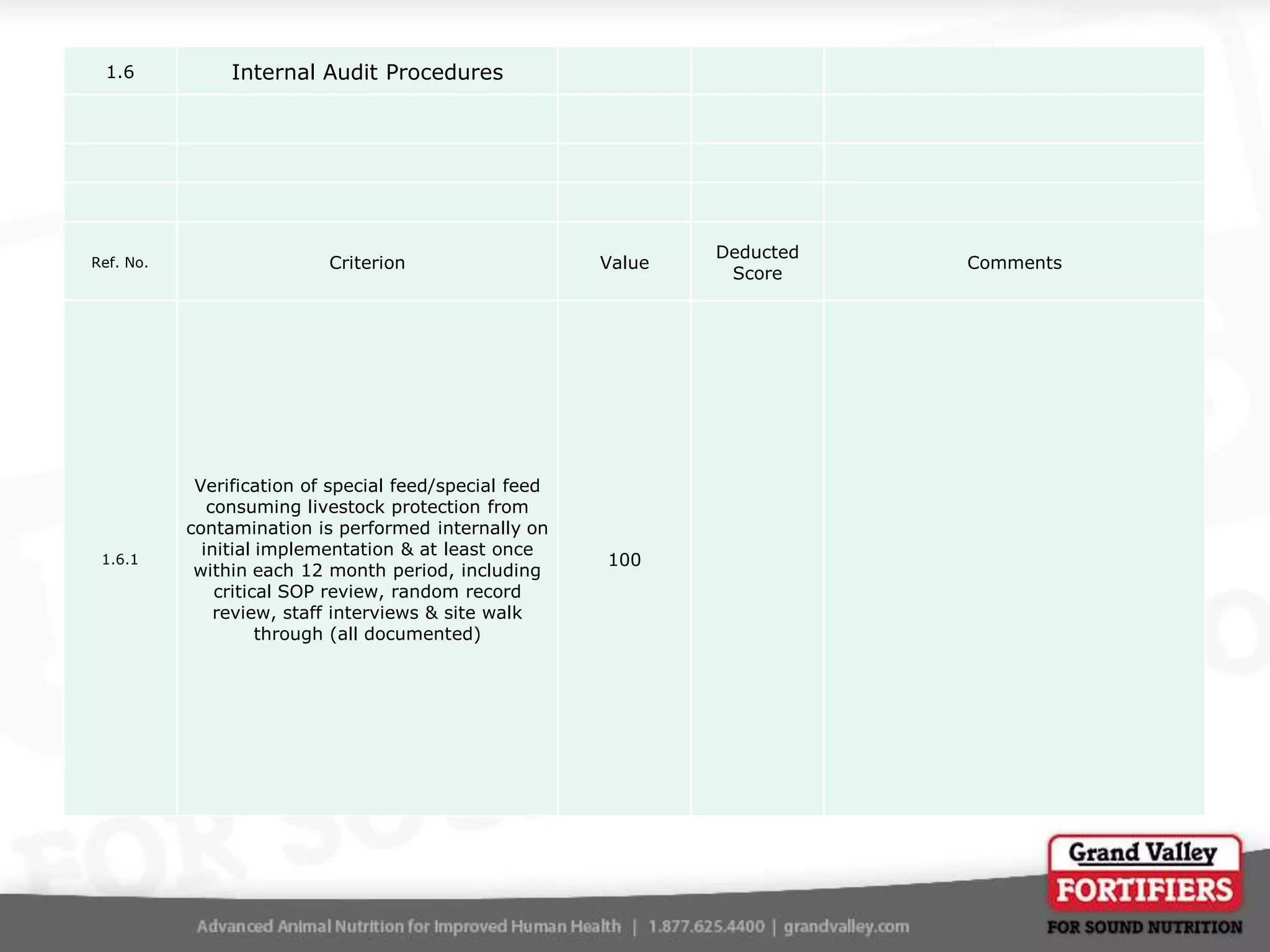
This document describes an audit process for facilities that produce special feed products without medications, animal by-products, or prohibited materials. It provides details on why audits are conducted, what an audit involves, how audits are conducted both internally and by third parties, and what standards facilities are audited against. The summary at the end indicates that the facility passed its audit with a score of 1000/1000, confirming its processes meet the audit standards for producing special feed products without restricted ingredients for at least the next 12 months.