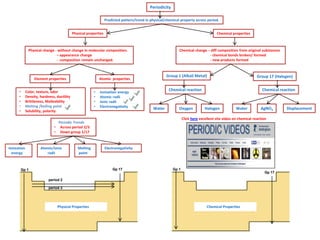
IB Chemistry on Chemical Properties, Oxides and Chlorides of period 3
- 1. Periodicity Predicted pattern/trend in physical/chemical property across period. Physical properties Chemical properties Physical change - without change in molecular composition. – appearance change - composition remain unchanged. Chemical change – diff composition from original substances - chemical bonds broken/ formed - new products formed Element properties Atomic properties • Color, texture, odor • Density, hardness, ductility • Brittleness, Malleability • Melting /boiling point • Solubility, polarity • Ionization energy • Atomic radii • Ionic radii • Electronegativity Periodic Trends • Across period 2/3 • Down group 1/17 Gp 1 Gp 17 period 3 period 2 Ionization energy Atomic/ionic radii Melting point Electronegativity Group 1 (Alkali Metal) Water Group 17 (Halogen) HalogenOxygen Chemical reaction Chemical reaction Water AgNO3 Displacement Click here excellent site video on chemical reaction Physical Properties Chemical Properties Gp 17 Gp 1
- 2. 2Li + CI2 -> 2LiCI 2Na + CI2 -> 2NaCI 2K + CI2 -> 2KCI Chemical Properties Group 1 Size increase Reaction with water 4Li + O2 -> 2Li2O 4Na + O2 -> 2Na2O 4K + O2 -> 2K2O Click here video potassium in water shell 2.1 2.8.1 2.8.8.1 2.8.8.18.1 Na Li K Rb lose electron easily electropositive Reactivity increase Group 1 (Alkali Metal) Chemical reaction 2Li + 2H2O -> 2LiOH + H2 2Na + 2H2O -> 2NaOH + H2 2K + 2H2O -> 2KOH + H2 Reaction with oxygen Reaction with halogen Lithium – move slowly surface water – red flame Sodium – move fast, hissing sound – yellow flame Potassium – move fast, ignite - lilac flame Turn red litmus blue- produce hydrogen gas Solution of metal hydroxide/alkaline produced Click here video sodium in water Similar chemical property but diff reactivity Lithium –burn slowly , red flame Sodium – burn brightly, yellow flame Potassium –burn very brightly, lilac flame Kept in paraffin oil Strong reducing agent Reduce H+ ion to H2 gas (losing e to H+)
- 3. F2 + 2KCI -> 2KF + CI2 CI2 + 2KBr -> 2KCI + Br2 Br2 + 2KI -> 2KBr + I2 Ag+ + CI- -> AgCI Ag+ + Br- -> AgBr Ag+ + I- -> AgI Chemical Properties Group 17 Size increase Reaction with water Click here video fluorine chemistry shell 2.7 2.8.7 2.8.8.7 2.8.18.18.7 CI F Br I Ability attract electron decrease/EN lower Reactivity decrease Group 17 (Halogen) Chemical reaction CI2 + H2O -> HCI + HOCI Br2 + H2O -> HBr + HOBr I2 + H2O -> HI + HOI Reaction with AgNO3 Adding AgNO3 AgCI – white ppt AgBr - yellow cream ppt AgI – yellow ppt Kept in seal, reactive Fluorine – yellow gas Chlorine – greenish gas Bromine – brown liquid Iodine – violet solid Click here video on chlorine chemistry Similar chemical property - decrease reactivity Chlorine – dissolve quickly –yellowish HOCI Bromine – dissolve slowly – brown HOBr Iodine – slightly soluble- brown HOI Displacement Reaction Reactive halogen displace less reactive halogen from its halide solution Click here video displacement reaction
- 4. Add NaBrAdd NaCIAdd NaIAdd NaCIAdd NaIAdd NaBr violet solid brown liquid yellow gas Click here video displacement rxn Click here video displacement rxn CI2 + 2NaBr -> 2NaCI + Br2 ✓ CI2 + 2NaI -> 2NaCI + I2 ✓ Br2 + NaCI -> ✗ Br2 + 2NaI -> 2NaBr + I2 ✓ I2 + NaCI -> ✗ I2 + NaBr -> ✗ Chemical Properties Group 17 Group 17 (Halogen) greenish gas Displacement Reaction Reactive halogen displace less reactive halogen from its halide solution CI2 in hexane Br2 in hexane I2 in hexane Br2 in hexane I2 in hexane I2 in hexane CI2 + 2NaBr -> 2NaCI + Br2 CI2 + 2NaI -> 2NaCI + I2 ✗ Br2 + 2NaI -> 2NaBr + I2 ✗ ✗
- 5. Na (metal) Mg (metal) AI (Metal) Si Non Metal P Non Metal S Non Metal CI Non Metal m/p (/C) 98 650 660 1423 44 120 -101 Conductivity Good Free e Good Free e Good Free e Moderate Semi Poor Molecular Poor Molecular Poor Molecular Bonding metallic metallic metallic Giant covalent Simple covalent Simple covalent Simple covalent Period 3 Across period 3 (Metal – Non metallic ) Na2O MgO AI2O3 SiO2 P4O10 P4O6 SO3 SO2 CI2O7 CI2O State Solid Solid Solid Solid Solid Solid Liquid Bonding Ionic Ionic Ionic Giant covalent Simple covalent Simple covalent Simple covalent m/p (C) High (1274) High (2852) High (2020) High (1610) Low (24) Low (17) Low (-92) Nature of Oxide Metal oxide Basic oxide Metal oxide Basic oxide Metal oxide Amphoteric Non metal oxide Acidic oxide Non metal oxide Acidic oxide Non metal oxide Acidic oxide Non metal oxide Acidic oxide Reaction with water Form NaOH (Alkaline) Form Mg(OH)2 (Alkaline) No reaction ✗ No reaction ✗ Form H3PO4 (Acidic) Form H2SO4 (Acidic) Form HCIO4 (Acidic) Oxides period 3 (Metal – Non metallic oxide ) Click here video oxides period 3 Water hydrolysis is chemical rxn Dissolving NOT a chemical rxn NaCI MgCI2 AI2CI6 SiCI4 PCI3 S2CI2 CI2 State Solid Solid Solid Liquid Liquid/Solid Liquid Gas Bonding Ionic Ionic Covalent Simple covalent Simple covalent Simple covalent Simple covalent m/p (C) High (801) High (714) Low (178) Low (-70) Low (-112) Low (-80) Low (-101) Conductivity Good Ions Good Ions Poor Poor Poor Poor Poor Reaction with water No reaction Dissolve ✗ No reaction Dissolve ✗ Water hydrolysis (Acidic) Water hydrolysis (Acidic) Water hydrolysis (Acidic) Water hydrolysis (Acidic) Water hydrolysis (Acidic) Click here video chloride period 3 Chloride period 3 (Metal – Non metallic chloride )
- 6. Hydrogen ion ((H+) produced Oxides period 3 (Metal – Non metallic ) Na2O MgO AI2O3 SiO2 P4O10 P4O6 SO3 SO2 CI2O7 CI2O Bonding Ionic Ionic Ionic Giant covalent Simple covalent Simple covalent Simple covalent Nature of Oxide Metal oxide Basic oxide Metal oxide Basic oxide Metal oxide Amphoteric Non metal oxide Acidic oxide Non metal oxide Acidic oxide Non metal oxide Acidic oxide Non metal oxide Acidic oxide Reaction with water Form NaOH (Alkaline) Form Mg(OH)2 (Alkaline) No reaction ✗ No reaction ✗ Form H3PO3 (Acidic) Form H2SO4 (Acidic) Form HCIO4 (Acidic) Chemical reaction - Water hydrolysis Breaking bond presence of water Na2O + H2O -> 2NaOH -> 2Na+ + 2OH- Metal /Basic oxide Ionic bonding Hydroxide ion ((OH-) produced Na2O -> 2Na+ + O2- Oxide ion :O:2- + H2O -> 2OH- H O H + - lone pair electron attract to H+ + H O H O 2- - : : Bond break pair electron move to oxygen - O O2- produce OH- become alkaline Water hydrolysis Non Metal /Acidic oxide covalent bonding Chemical reaction - Water hydrolysis Breaking bond presence of water SO2 + H2O -> H2SO3 Reaction mechanism Reaction mechanism O O S - - + Lone pair electron attract to S+ Bond polarity of SO2 cause hydrolysis H2O O H H S O O O H H Bond break O O O H S H H2SO3 H2SO3 -> 2H+ + SO3 2- - H+ makes it acidic
- 7. H2SO4H2SO4 -> 2H+ + SO4 2- - H+ makes it acidic Hydrogen ion ((H+) produced Oxides period 3 (Metal – Non metallic ) Na2O MgO AI2O3 SiO2 P4O10 P4O6 SO3 SO2 CI2O7 CI2O Bonding Ionic Ionic Ionic Giant covalent Simple covalent Simple covalent Simple covalent Nature of Oxide Metal oxide Basic oxide Metal oxide Basic oxide Metal oxide Amphoteric Non metal oxide Acidic oxide Non metal oxide Acidic oxide Non metal oxide Acidic oxide Non metal oxide Acidic oxide Reaction with water Form NaOH (Alkaline) Form Mg(OH)2 (Alkaline) No reaction ✗ No reaction ✗ Form H3PO3 (Acidic) Form H2SO4 (Acidic) Form HCIO4 (Acidic) Chemical reaction - Water hydrolysis Breaking bond presence of water MgO + H2O -> 2MgOH -> 2Mg+ + 2OH- Metal /Basic oxide Ionic bonding Hydroxide ion ((OH-) produced MgO -> Mg2+ + O2- Oxide ion :O:2- + H2O -> 2OH- H O H + - lone pair electron attract to H+ + H O H O 2- - : : Bond break pair electron move to oxygen - O O2- produce OH- become alkaline Water hydrolysis Non Metal /Acidic oxide covalent bonding Chemical reaction - Water hydrolysis Breaking bond presence of water SO3 + H2O -> H2SO4 Reaction mechanismReaction mechanism O O S - - + Lone pair electron attract to S+ Bond polarity of SO3 cause hydrolysis H2O O H H S O O O H H Bond break O O O H S H O O O
- 8. Written in two waysWritten in two ways Na2O MgO AI2O3 SiO2 P4O10 P4O6 SO3 SO2 CI2O7 CI2O Bonding Ionic Ionic Ionic Giant covalent Simple covalent Simple covalent Simple covalent Nature of Oxide Metal oxide Basic oxide Metal oxide Basic oxide Metal oxide Amphoteric Non metal oxide Acidic oxide Non metal oxide Acidic oxide Non metal oxide Acidic oxide Non metal oxide Acidic oxide Reaction with water Form NaOH (Alkaline) Form Mg(OH)2 (Alkaline) No reaction ✗ No reaction ✗ Form H3PO4 (Acidic) Form H2SO4 (Acidic) Form HCIO4 (Acidic) Amphoteric oxide + OH - Act as acid 3+ High charge density AI3+ attract lone pair e of OH- Act as base AI2O3 + 3H2O or 2AI(OH)3 AI2O3 + 3H2O or 2AI(OH)3 3+ OH - As acid – react with base AI2O3 + 3H2O + 2OH- -> 2AI(OH)4 - 2AI(OH)3 + 2OH- -> 2AI(OH)4 - As base – react with acid Al2O3 + 6HCI -> 2AICI3 + 3H2O 2AI(OH)3 + 6H+ -> 2AI3+ + 6H2O H H H H H H + + + + + + AI AI H2O H2O H2O H2O H2O H2O 3+ 3+ OH- react with H+ form H2O Water hydrolysis AI2O3 no reaction with water BUT reacts with either acid or base - -
- 9. Strong covalent bonds H3PO3 P4O6 + 6H2O -> 4H3PO3 Hydrogen ion ((H+) produced Na2O MgO AI2O3 SiO2 P4O10 P4O6 SO3 SO2 CI2O7 CI2O Bonding Ionic Ionic Ionic Giant covalent Simple covalent Simple covalent Simple covalent Nature of Oxide Metal oxide Basic oxide Metal oxide Basic oxide Metal oxide Amphoteric Non metal oxide Acidic oxide Non metal oxide Acidic oxide Non metal oxide Acidic oxide Non metal oxide Acidic oxide Reaction with water Form NaOH (Alkaline) Form Mg(OH)2 (Alkaline) No reaction ✗ No reaction ✗ Form H3PO3 (Acidic) Form H2SO4 (Acidic) Form HCIO4 (Acidic) No reaction with water ✗ Non metal oxide Giant macromolecular structure SiO2 + 2NaOH -> Na2SiO3 + H2O SiO2 + 2OH- -> SiO3 2- + H2O O O OSi Bond break - Si Non Metal/Acidic oxide covalent bonding Chemical reaction - Water hydrolysis Breaking bond presence of water Lone pair electron attract to P+ Bond polarity of P4O6 cause hydrolysis H2O O H H P O O H H Bond break O O H P H H2PO3 -> 3H+ + PO3 2- - H+ makes it acidic O O H As acid – react with base O H Lone pair electron attract to Si+ O O H O - Water hydrolysis SiO2 react with OH- -> SiO3 2- H SiO2 no reaction with water BUT reacts with base
- 10. Hydrogen ion ((H+) produced NaCI(s) + H2O -> NaCI (aq) MgCI2(s) + H2O -> MgCI2 (aq) Ionic compound Ionic bonding Ions are hydrated by water molecules Ion attract polar H2O Simple covalent covalent bonding Chemical reaction - Water hydrolysis Breaking bond presence of water SiCI4 + 4H2O -> Si(OH)4 + 4HCI Reaction mechanism show only ONE H2O CI Si - - + Lone pair electron attract to Si+ Bond polarity of SiCI4 cause hydrolysis of H2O O H H Si CI O H Bond break H+ makes it acidic NaCI MgCI2 AI2CI6 SiCI4 PCI3 S2CI2 CI2 Bonding Ionic Ionic Covalent Simple covalent Simple covalent Simple covalent Simple covalent Conductivity Good Ions Good Ions Poor Poor Poor Poor Poor Reaction with water No reaction (Neutral) ✗ No reaction (Neutral) ✗ Water hydrolysis (Acidic) Water hydrolysis (Acidic) Water hydrolysis (Acidic) Water hydrolysis (Acidic) Water hydrolysis (Acidic) Dissolving - NO chemical rxn - NO water hydrolysis Na+ CI- Mg2+ CI- CI-Mg2+ CI CI CI - - CI CI CI H Si CI CI CI O H + H + Water hydrolysis Chloride period 3 (Metal – Non metallic chloride) NaCI/MgCI2 no reaction with water
- 11. AICI3 Bond breaking AICI3 + 3H2O -> AI(OH)3 + 3HCI Water hydrolysis Lone pair electron on oxygen attract to AI3+ NaCI MgCI2 AI2CI6 SiCI4 PCI3 S2CI2 CI2 Bonding Ionic Ionic Covalent Simple covalent Simple covalent Simple covalent Simple covalent Conductivity Good Ions Good Ions Poor Poor Poor Poor Poor Reaction with water No reaction (Neutral) ✗ No reaction (Neutral) ✗ Water hydrolysis (Acidic) Water hydrolysis (Acidic) Water hydrolysis (Acidic) Water hydrolysis (Acidic) Water hydrolysis (Acidic) CI covalent bonding AI3+ion - small size, + high charge – high charge density H H O + + AI AI CI CI CI CI CI CI Covalent and ionic character AI2CI6 High charge density AI3+ attract lone pair electron from CI form dative bond 3+ 3+ High charge density AI3+ hydrated by six H2O AI3+ CI CI - - - Water hydrolysis -breaking bond presence of water AICI3 is acidic Polarise H2O -release of H+ – ACIDIC AI3+ + CI CI CI - - - AI3+ : : H H H H H H O O O AI3+ H O O H H H + + High charge density AI3+ hydrated by six H2O H+ makes it acidic Chloride period 3 (Metal – Non metallic chloride) AICI3 written as AI(H2O)6 3+ + 3CI-
- 12. NaCI MgCI2 AI2CI6 SiCI4 PCI3 S2CI2 CI2 Bonding Ionic Ionic Covalent Simple covalent Simple covalent Simple covalent Simple covalent Conductivity Good Ions Good Ions Poor Poor Poor Poor Poor Reaction with water No reaction (Neutral) ✗ No reaction (Neutral) ✗ Water hydrolysis (Acidic) Water hydrolysis (Acidic) Water hydrolysis (Acidic) Water hydrolysis (Acidic) Water hydrolysis (Acidic) H+ makes it acidic SiCI4 + 4H2O -> Si(OH)4 + 4HCI Chemical reaction - Water hydrolysis Breaking bond presence of water Hydrogen ion ((H+) produced Simple covalent covalent bonding Chemical reaction - Water hydrolysis Breaking bond presence of water PCI3 + 3H2O -> P(OH)3 /H3PO3 + 3HCI CI Si - - + Lone pair electron attract to Si+ Bond polarity of SiCI4 cause hydrolysis H2O O H H Si CI O H Bond break H+ makes it acidic CI CI CI - - CI CI CI H Si CI CI CI O H + H + Simple covalent covalent bonding Hydrogen ion ((H+) produced P H + CI CI CI O H H P CI CI CI O H H Bond break P O CI CI H + + Chloride period 3 (Metal – Non metallic chloride) Water hydrolysis Reaction mechanism show only ONE H2O Reaction mechanism show only ONE H2O
- 13. Lone pair electron attract to Si+ Bond polarity of SiCI4 cause hydrolysis H2O H- H H Bond break Si H + Reaction mechanism show ONE H2O Reaction mechanism show ONE H2O Reaction mechanism show FOUR H2O Reaction mechanism show THREE H2O Si O O O O H H H H H H H CI CI CI CI O O O O H H H H SiCI4 + 4H2O -> Si(OH)4 + 4HCI - - - P O O O H H H H CI CI CI Lone pair electron attract to P+ Bond polarity of PCI3 cause hydrolysis H2O Bond break + O O O P H H PCI3 + 3H2O -> P(OH)3 /H3PO3 + 3HCI + + CI CI CI CI CI CI CI - - - - - - - - - -
