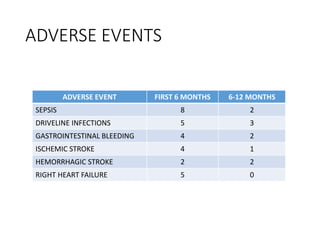
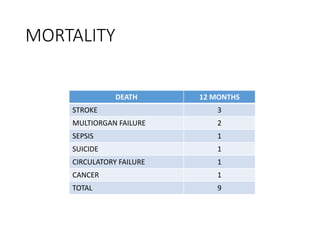
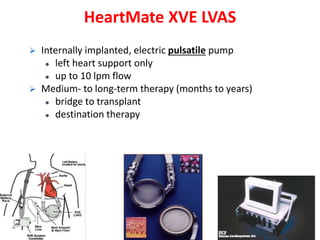
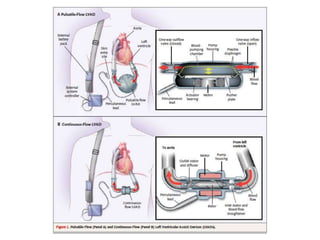
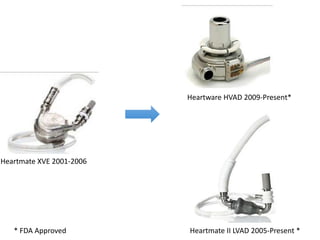
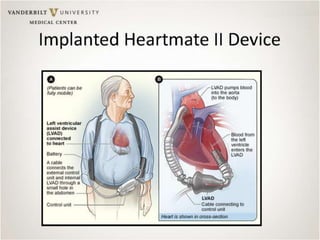
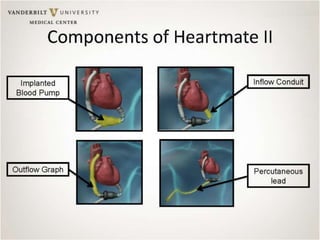
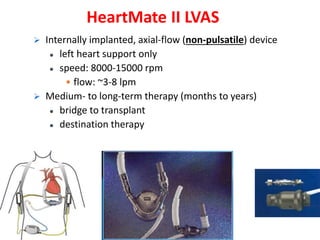
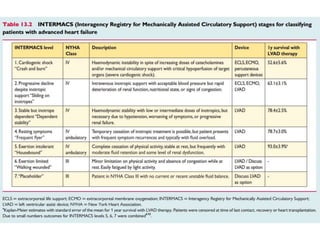
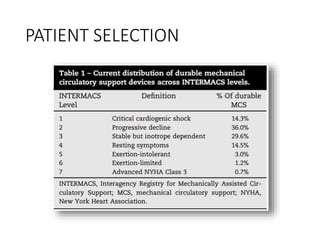
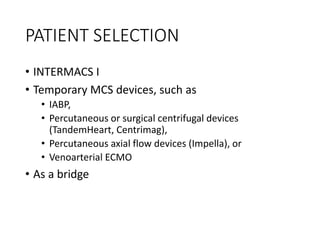
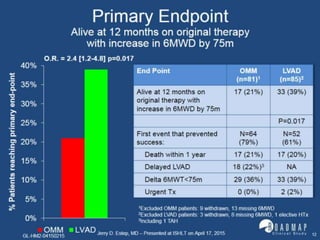
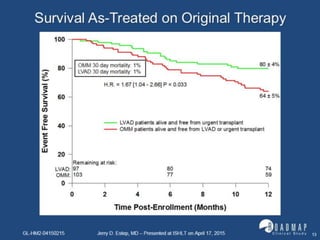
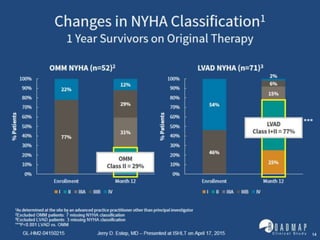
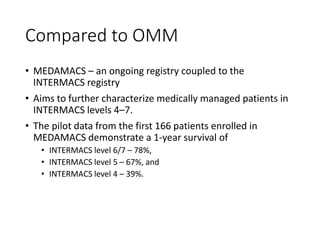
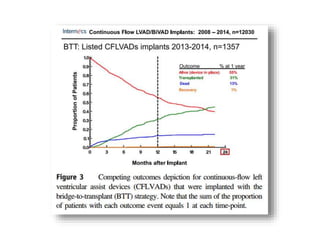
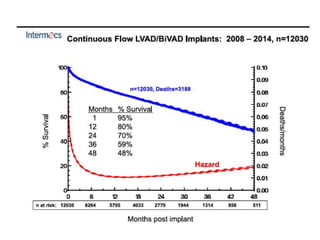
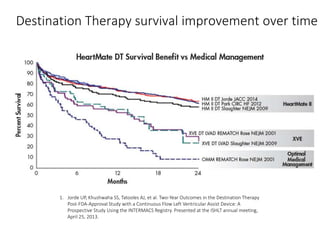
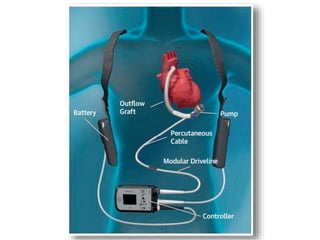
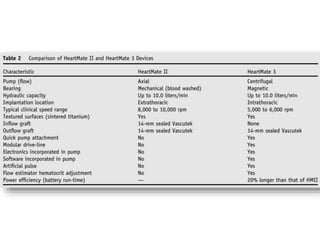
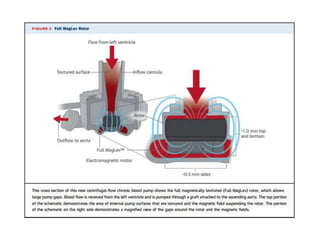
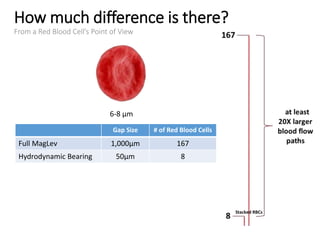
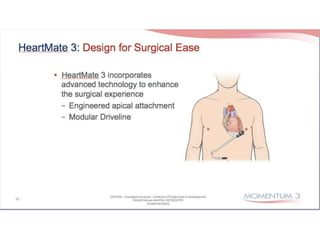
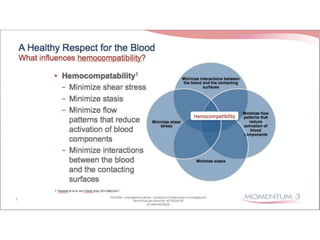
The document discusses mechanical circulatory support systems, particularly durable left ventricular assist devices (LVADs) like HeartMate II and HeartWare, highlighting their design, function, therapy applications, and patient selection criteria. It also presents data on patient outcomes, complications, and potential benefits, like improved survival rates and quality of life for those with advanced heart failure, alongside addressing complications and contraindications associated with LVAD implantation. Additionally, the document examines emerging technologies and recent clinical trials, particularly focusing on patient demographics, performance metrics, and long-term survival data.































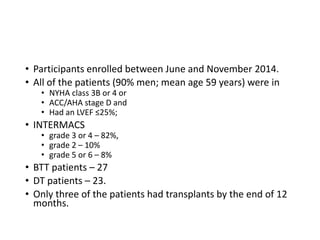

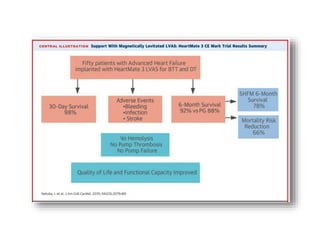

![• Survival rate at 6 months – 92%
• European CE Mark approval in October 2015.
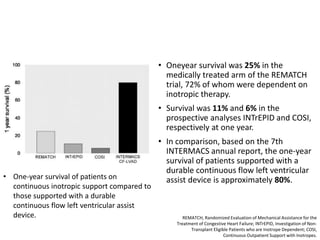
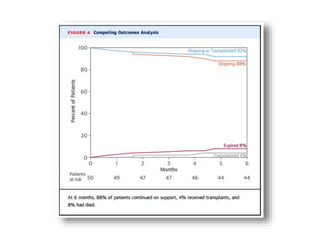
• Survival rate at 1 year – 80%
[combining destinationtherapy (DT) and bridgetotransplant (BTT)]
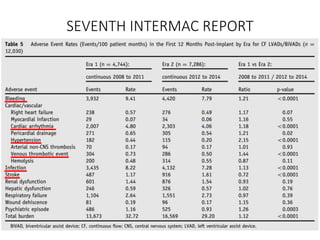
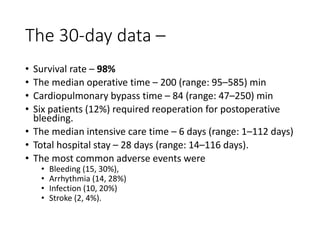
• There were no occurrences of pump thrombosis, malfunctions, or
hemolysis.
• Both gastrointestinal (GI) bleeding and strokes were reduced by 50%
between the 6month and 1year assessment points.
• There were four GI events during the first 6 months, but only two
additional events occurred in the second 6 months.
• Six strokes of any type occurred during the first 6 months vs three
additional strokes during the second.
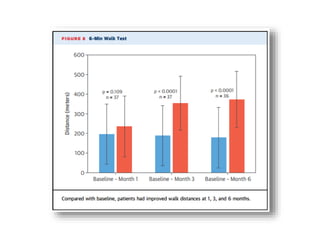
• The 6minute walk test increased significantly from baseline, as did
quality of life scores (both comparisons, P<0.0001).
Krabatsch T, Schmitto JD, Pya Y, et al. HeartMate 3 fully magnetically levitated left ventricular assist device for the treatment of advanced HF—1year
results from the CE Mark Trial. International Society for Heart and Lung Transplantation 2016 Scientific Sessions; April 28, 2016; Washington, DC](https://image.slidesharecdn.com/lvad-170405061156/85/LVAD-Left-Ventricular-Assist-Device-85-320.jpg)