F block element
•Download as PPTX, PDF•
28 likes•24,718 views
based on maharashtra board 2015 syllabus.
Report
Share
Report
Share
Recommended
Recommended
More Related Content
What's hot
What's hot (20)
REDOX REACTION : inner & outer sphere Complimentary & non-complimentary reaction

REDOX REACTION : inner & outer sphere Complimentary & non-complimentary reaction
Similar to F block element
Similar to F block element (20)
F Block Elements and characteristics of lanthanides.pptx

F Block Elements and characteristics of lanthanides.pptx
5th Lecture on Transition & Inner Transition Elements | Chemistry Part I | 12...

5th Lecture on Transition & Inner Transition Elements | Chemistry Part I | 12...
Lanthanide and actinide chemistry, inorganic chemistry. inner transistion ser...

Lanthanide and actinide chemistry, inorganic chemistry. inner transistion ser...
CHEMISTRY OF F-BLOCK ELEMENTS BY K.N.S.SWAMI..pdf473.pdf.pdf

CHEMISTRY OF F-BLOCK ELEMENTS BY K.N.S.SWAMI..pdf473.pdf.pdf
Class 11 chapter 3 Cassification of elements and periodicity in properties ppt

Class 11 chapter 3 Cassification of elements and periodicity in properties ppt
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES

CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
More from nysa tutorial
More from nysa tutorial (20)
10._magnetic_effect_due_to_electric_current_phy_12_2019.pptx

10._magnetic_effect_due_to_electric_current_phy_12_2019.pptx
Recently uploaded
God is a creative God Gen 1:1. All that He created was “good”, could also be translated “beautiful”. God created man in His own image Gen 1:27. Maths helps us discover the beauty that God has created in His world and, in turn, create beautiful designs to serve and enrich the lives of others.
Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...

Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...christianmathematics
This presentation was provided by William Mattingly of the Smithsonian Institution, during the fourth segment of the NISO training series "AI & Prompt Design." Session Four: Structured Data and Assistants, was held on April 25, 2024.Mattingly "AI & Prompt Design: Structured Data, Assistants, & RAG"

Mattingly "AI & Prompt Design: Structured Data, Assistants, & RAG"National Information Standards Organization (NISO)
Recently uploaded (20)
Web & Social Media Analytics Previous Year Question Paper.pdf

Web & Social Media Analytics Previous Year Question Paper.pdf
Kisan Call Centre - To harness potential of ICT in Agriculture by answer farm...

Kisan Call Centre - To harness potential of ICT in Agriculture by answer farm...
Interactive Powerpoint_How to Master effective communication

Interactive Powerpoint_How to Master effective communication
Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...

Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...
9548086042 for call girls in Indira Nagar with room service

9548086042 for call girls in Indira Nagar with room service
A Critique of the Proposed National Education Policy Reform

A Critique of the Proposed National Education Policy Reform
Mattingly "AI & Prompt Design: Structured Data, Assistants, & RAG"

Mattingly "AI & Prompt Design: Structured Data, Assistants, & RAG"
Measures of Central Tendency: Mean, Median and Mode

Measures of Central Tendency: Mean, Median and Mode
F block element
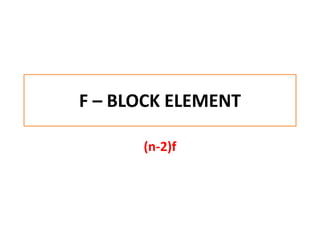
- 1. F – BLOCK ELEMENT (n-2)f
- 2. Introduction • Penultimate (n -1)d • prepenultimate (n -2)f • Inner transition element- e- use is both shell. • Intermediate behavior like d- block element. • 2 series of f- block element- • Lanthanide series – our main topic. • actinide series- radioactive.
- 3. Lanthanide series • La(57) Ce(58) Pr(59) • Nd(60) Pm(61) Sm(62) • Eu(63) Gd(64) Tb(65) • Dy(66) Ho(67) Er(68) • Tm(69) Yb(70) Lu(71) • 14 element is called lanthanide series. • General electronic configuration 4f1-14 5d0-1 6s2
- 4. Ln Greek hidden Lanthanum, a “rare earth” is used in camera lenses amongst other things. Lanthanum is found in uranium minerals
- 5. Cerium Sparking light. Also used in permanent magnet.
- 6. Praseodymium
- 7. Praseodymium
- 8. Neodymium
- 10. Neodymium
- 11. Europeium
- 12. • Europium is used to produce blue, red and white radiances in computer monitors and television screens. It is also used in energy efficient light bulbs.
- 13. Position of lanthanoids 13 6 7 57 -71 89-103 Total= 14 element Same no. of valency 5d1 and 6s2. Same chemical & physical property. Fill orbital 4f 5f
- 14. Electronic configuration of lanthanoids • E.C Based on aufbau principle . La (57) 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 5d1 6s2 [Xe] – 54 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 The electronic configuration of Ln is [Xe] 4f0 5d1 6s2 or [Xe] 4f0 Similarly Ce- 4f1, Pr- 4f2,……… Lu-4f14.(expected) but observed E.C is not true for all element.
- 16. Oxidation state • Common oxidation state is +3 more stable. • Loses of two 6s electron and one 5d or 4f electron. • Some either show +2 or +4 Oxidation state. • Ln [Xe] 4f0 5d1 6s2 and Ln+3 [Xe]4f0 5d0 6s0 Ln, Pm, Ho, Eb, Lu +3 Ce, Pr, Tb, Dy +3, +4 Sm, Eu, Tm, Yb +2, +3 Nd, +2, +3, +3
- 17. Chemical reactivity of Lanthanids 1. Carbides-C 2. Hydrides- H2 3. Oxides- O2 4. Reaction with nitrogen 5. Reaction with mineral acids 6. Reaction with water 7. Reaction with sulphure
- 18. • The first few members of the lanthanoids are quite reactive and show chemical behaviour similar to that of calcium. The standard electrode potentials of the Ln+3/Ln couple indicate that all the lanthanides are more reactive than aluminium. • These metals combine with hydrogen when heated gently in the gas, and form hydrides of the type LnH2 and LnH3. Chemical reactivity of Lanthanids
- 19. • lanthanoids (Ln) Ln + 3H2 → 2LnH3 lanthanoid Hydrogen Lanthanoid hydride 2Ln + 3O2 → 2Ln2O3 lanthanoid Oxygen Lanthanoid Oxide • The oxide Ln2O3 react with water to form insoluble hydroxides. Ln2O3 + 3H2O → 2Ln(OH)3 Ln2O3 + 3CO2 → Ln2(CO3)3 Chemical reactivity of Lanthanids
- 20. • On being heated, these elements combine directly with non-metals, and form carbides with carbon, nitrides with nitrogen, sulphides with sulphur, and halides with halogens. • 2773K Ln + 2C → 2LnC2 lanthanoid carbon Carbide • Δ 2Ln + N2 → 2LnN lanthanoid Nitrogen Nitride Δ 2Ln + 3S → 2Ln2S3 lanthanoid Sulphur Sulphide Chemical reactivity of Lanthanids
- 21. • 2Ln + 3H2O → 2Ln(OH)3 + 3H2 lanthanoid water Halide • They liberate hydrogen from dilute acids. Δ 2Ln + 6HX → 2LnX3 + 3H2↑ lanthanoid Nitrogen Halide hydrogen Chemical reactivity of Lanthanids
- 22. Lanthanoid contraction and its consequences
- 23. Lanthanoid contraction • The gradual decrease in atomic and ionic size of lanthanoides with increase in atomic number is called lanthanoide contraction. • Causes of Lanthanoid contraction- • As atomic no. increase the positive charge also increase. • As atomic no. increase other thing decreases • Atomic radius= ionic radius > screen effect.
- 24. Effects of Lanthanoid contraction a) Decrease in basicity-(fajan’s principle) Larger the size of cation, greater is the tendency of such hydroxides to dissociate(weak bond) and stronger will be the base(Covalent nature).
- 25. • b) Ionic radii of post lanthanoids • The element which follow the lanthanoids in the third transition series are known as post lanthanoids. • The ionic radii of the element which follow lanthanium(Hf, Ta, W, etc) are similar to that of the element of previous period. • There is normal increase in size Sc to Y to La. Effects of Lanthanoid contraction
- 26. Group Series ↓ 4 5 6 7 1st transition Ti(132pm) V(122pm) Cr(106pm) Mn(94pm) 2nd transition Zr(145pm) Nb(134pm) Mo(129pm) Tc(114pm) 3rd transition Hf(144pm) Ta(134pm) W(130pm) Re(114pm) Effects of Lanthanoid contraction This are similar no. of valency and property is called chemical twins.
- 27. Actinoids • 7 period and actinide series. • Electron enter in 5f orbital. • Many physical and chemical property are similar to actinium(actinoids). • Second inner transition element. • Outermost and penultimate shell remain the same. • General E.C 5f1-10 6d0-1 7s2
- 28. Actinide • Np(93)- Neptune Pu(94)
- 29. Trans-uranic element • Manmade element atomic no. higher then uranium-92.
- 30. Electronic configuration • Ac (89)- 1s22s22p63s23p63d104s24p64d105s25p64f145d106s26 p66d17s2. • [Rn]- 86 • 1s22s22p63s23p63d104s24p64d105s25p64f145d106s26 p6 • Ac electronic configuration is • [Rn] 6d17s2 or [Rn] 5f0 6d1 7s2 • 6d and 7s electron is same only 5f electron change by increasing order. Th(90)- [Rn] 5f1,…….. 5f10.
- 32. Oxidation state of actinoids • Common oxidation state is +3. • Np+3, Pu+3 get oxidised Np+4,Pu+4 in aqs soln. some become stable some unstable. Ac Th Pa U Np Am Cm Bk Cf Es Fm Md No Lr 2 2 2 3 3 3 3 3 3 3 3 3 3 3 3 3 3 4 4 4 4 4 4 4 4 5 5 5 5 5 6 6 6 6 7 7
- 33. Comparison between lanthanoids and actinoids
Editor's Notes
- Oxocations are the polyatomic cations contain one or more oxygen atoms.Example: VO +, VO2 +, TO2 + etc Binding energy- molecuel ko combine hone me jo energy kharch hoti hair use B.E.
