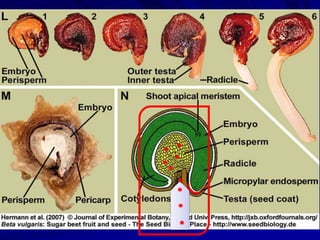
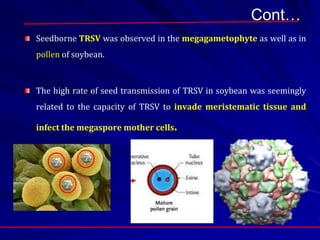
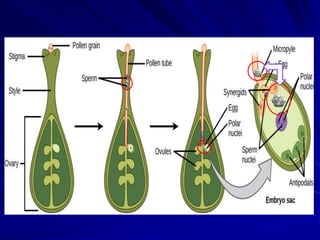
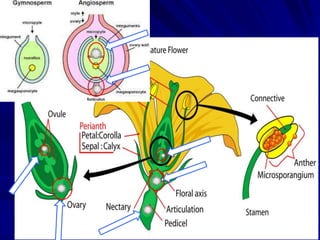
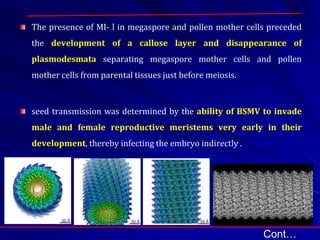
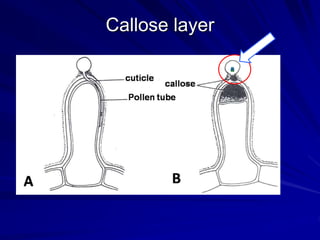
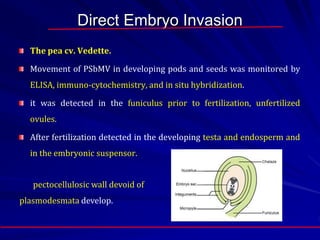
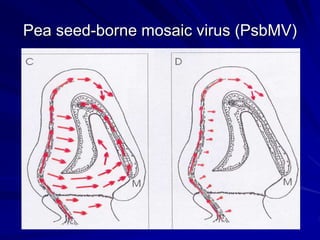
The document discusses various modes of virus entry into seeds, highlighting both direct and indirect infection methods, as well as the genetic and physiological aspects of seed transmission. It traces the historical context of seed-borne viruses and their longevity in seeds, alongside specific examples of viruses affecting agricultural crops. The findings underscore the complexities of virus transmission, including the role of reproductive tissues and environmental factors during seed development.