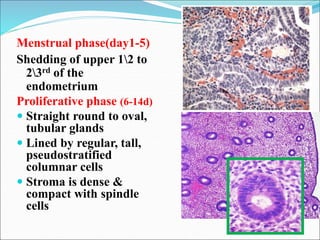
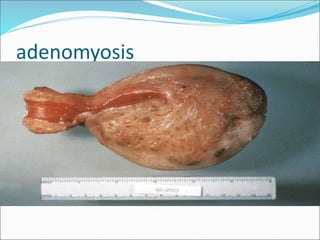
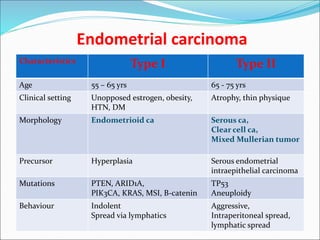
The document discusses the normal structure and function of the uterus, as well as several uterine disorders including dysfunctional uterine bleeding, endometriosis, adenomyosis, endometrial hyperplasia, and endometrial carcinoma. It provides details on the etiology, pathogenesis, morphology, clinical features, and staging of these conditions. Key points include that the uterus contains a myometrium and endometrium, and the endometrium undergoes monthly changes regulated by hormones. Dysfunctional uterine bleeding is abnormal bleeding without structural abnormalities caused by hormonal disturbances. Endometriosis and adenomyosis involve the presence of endometrial tissue outside or within the uterus, respectively. Endometrial hyperplasia and