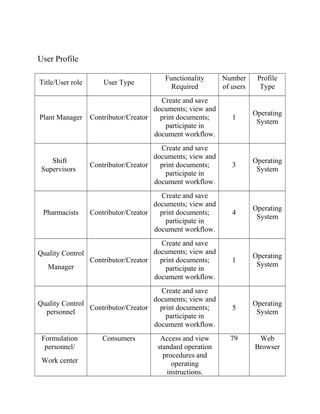
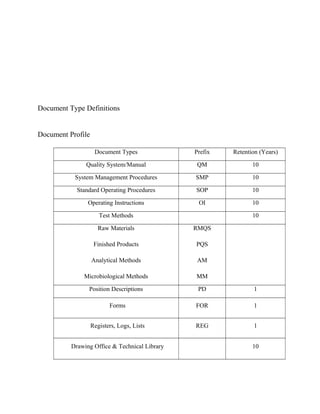
This document outlines user requirements for a document management system for Health First Pharmaceutical Company's Manufacturing Division. It defines requirements for document submission, review, approval, metadata migration from current systems, and record retention. The system must support ISO and cGMP compliance. Key user types are identified along with their access levels and needs. Document library structure and metadata requirements are also specified.