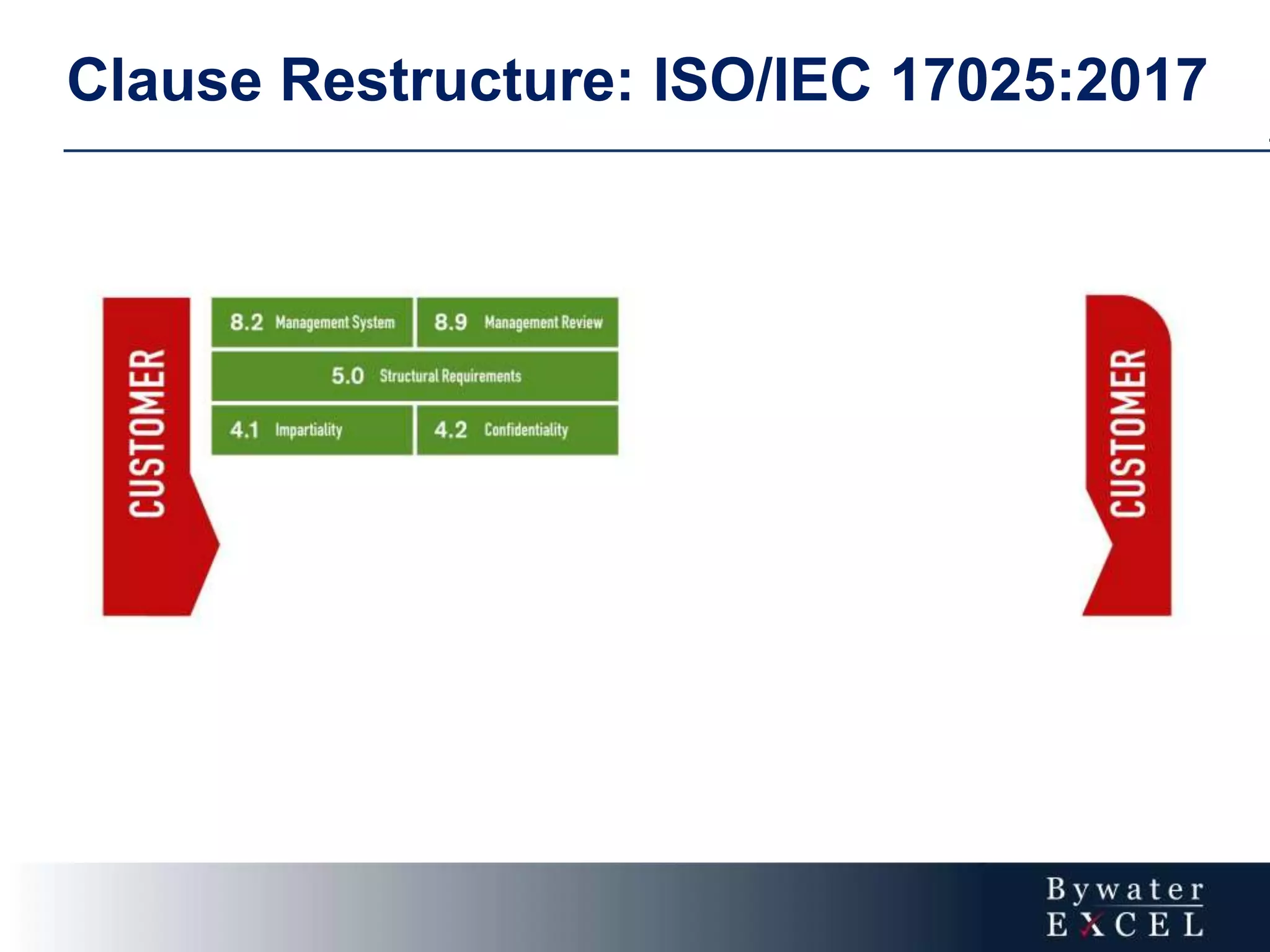
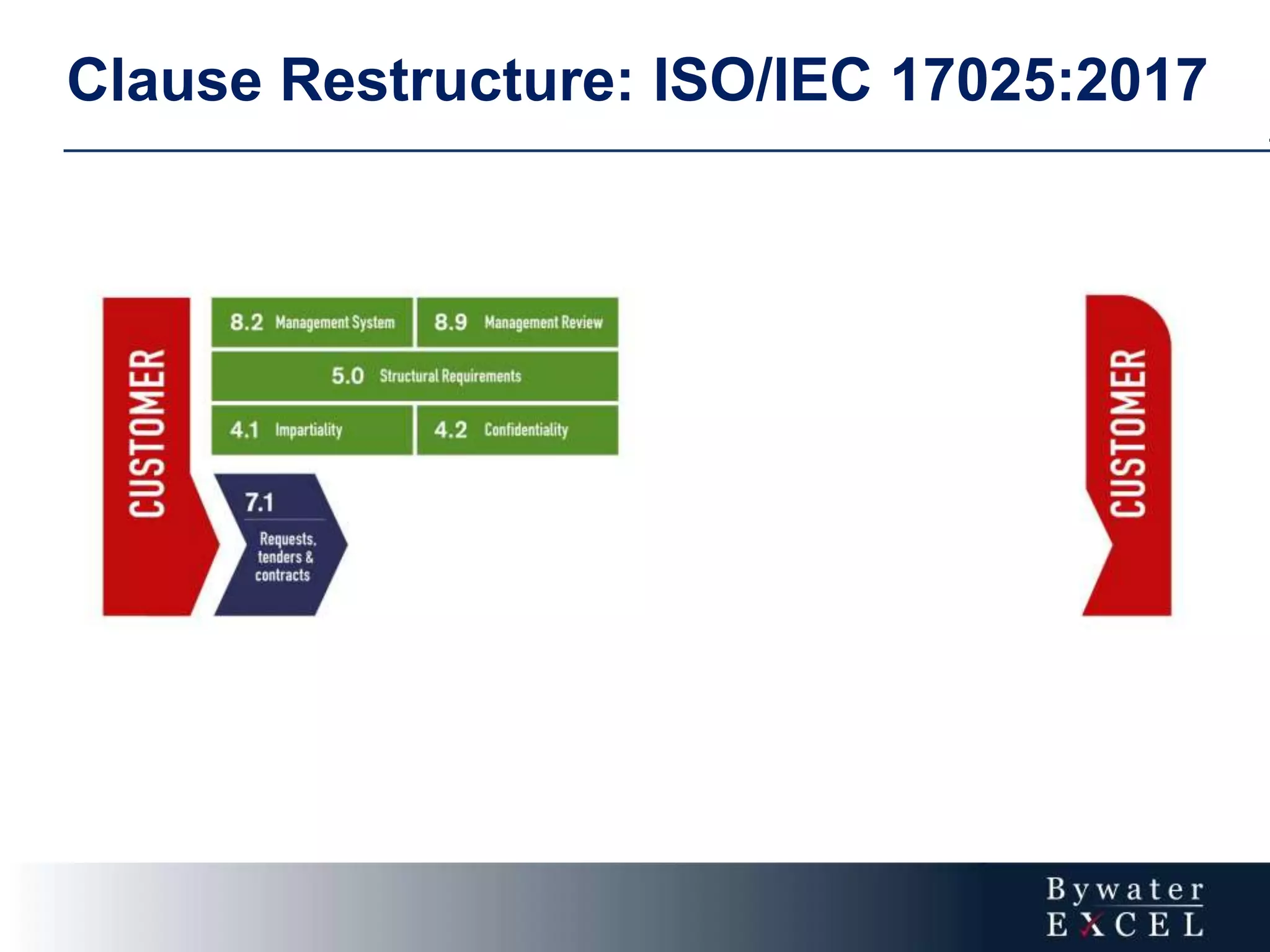
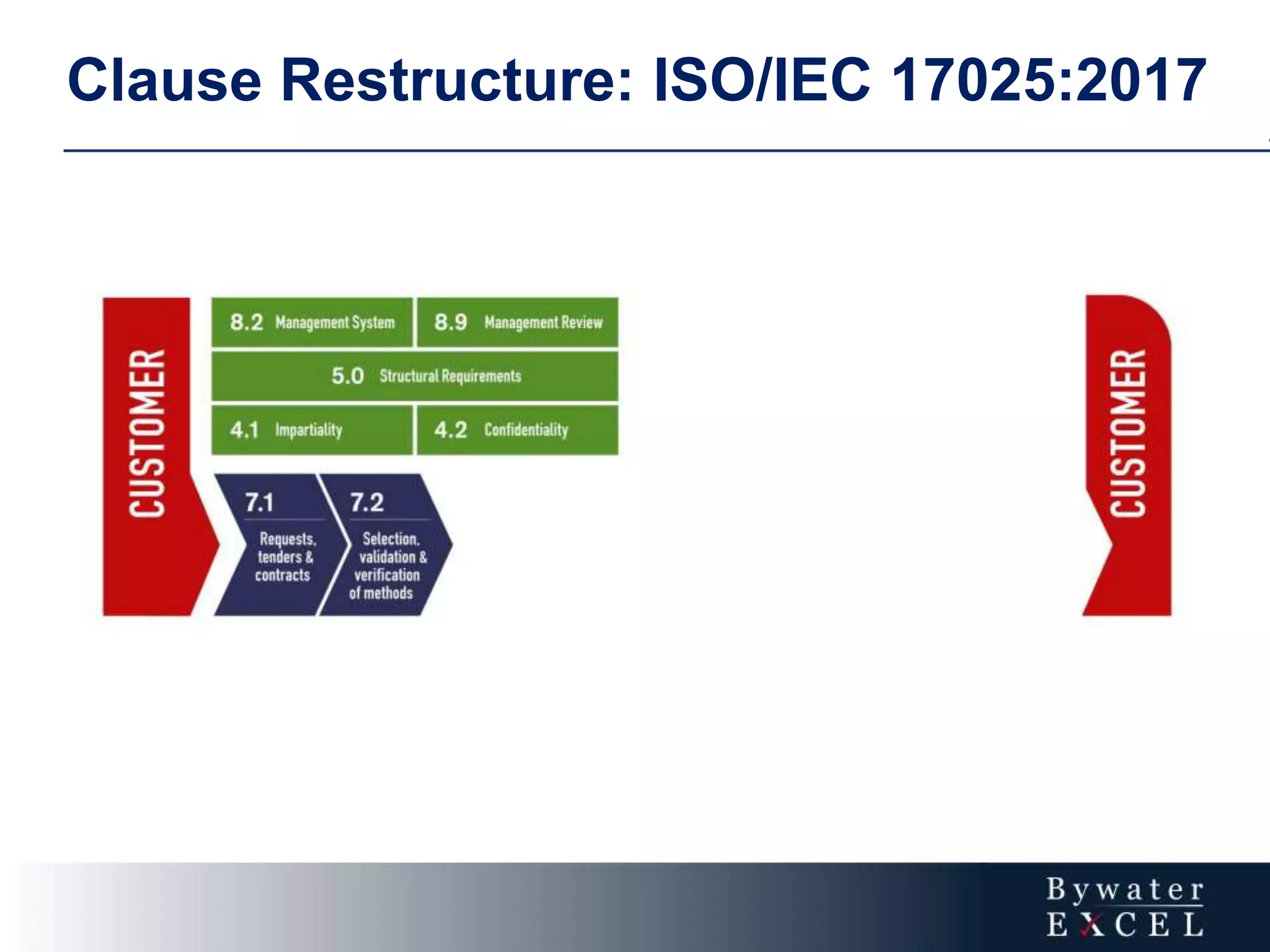
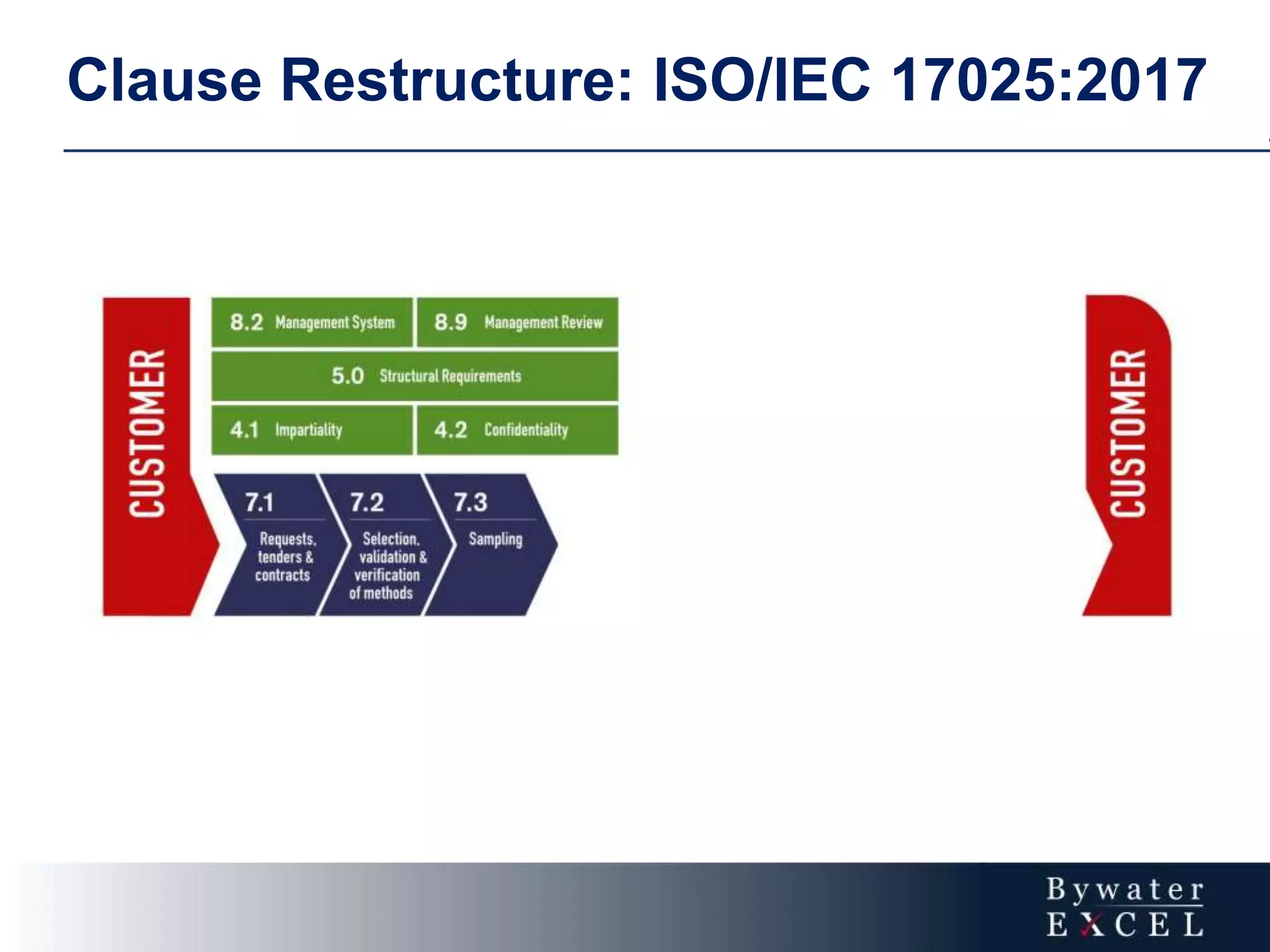
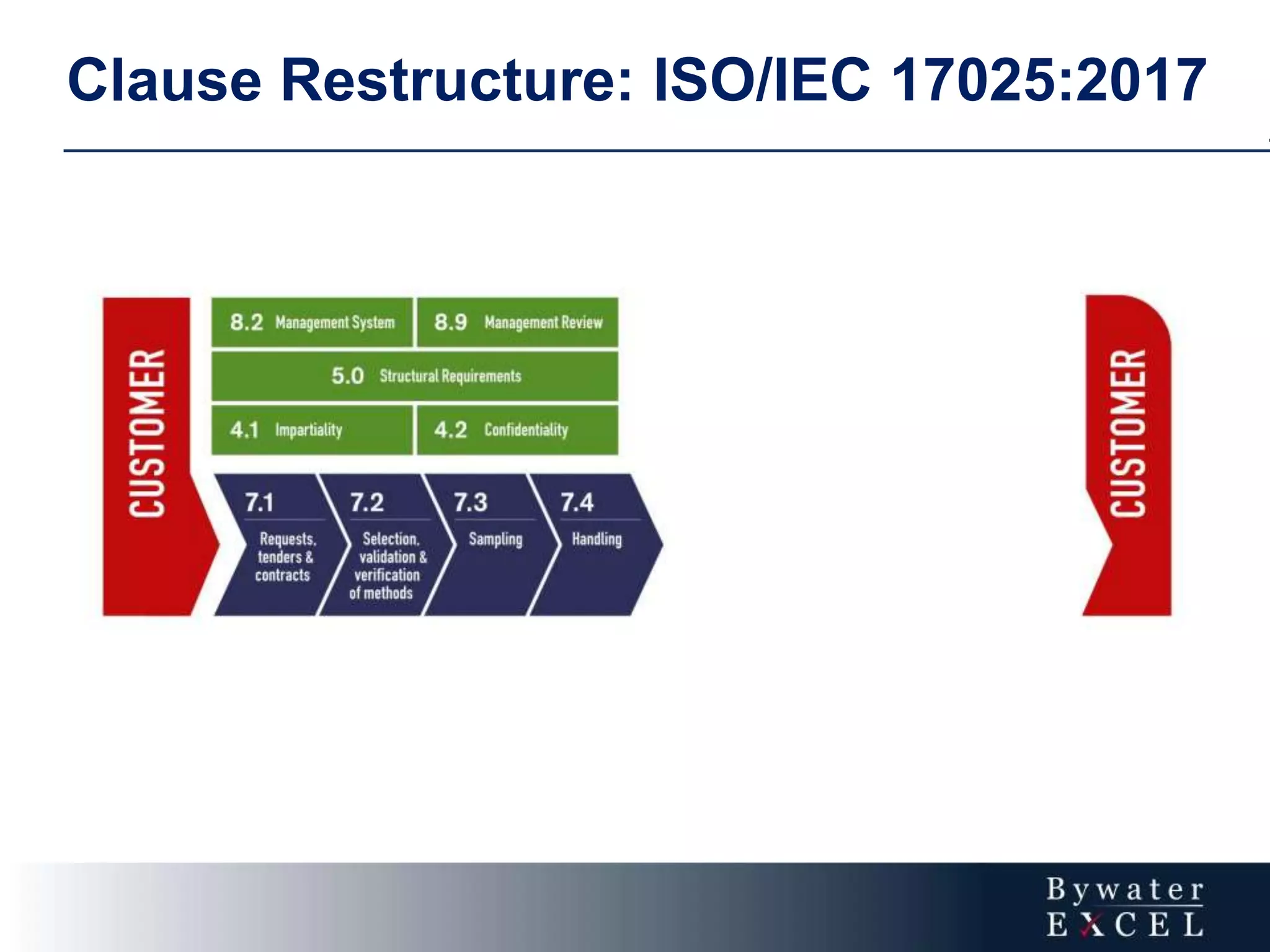
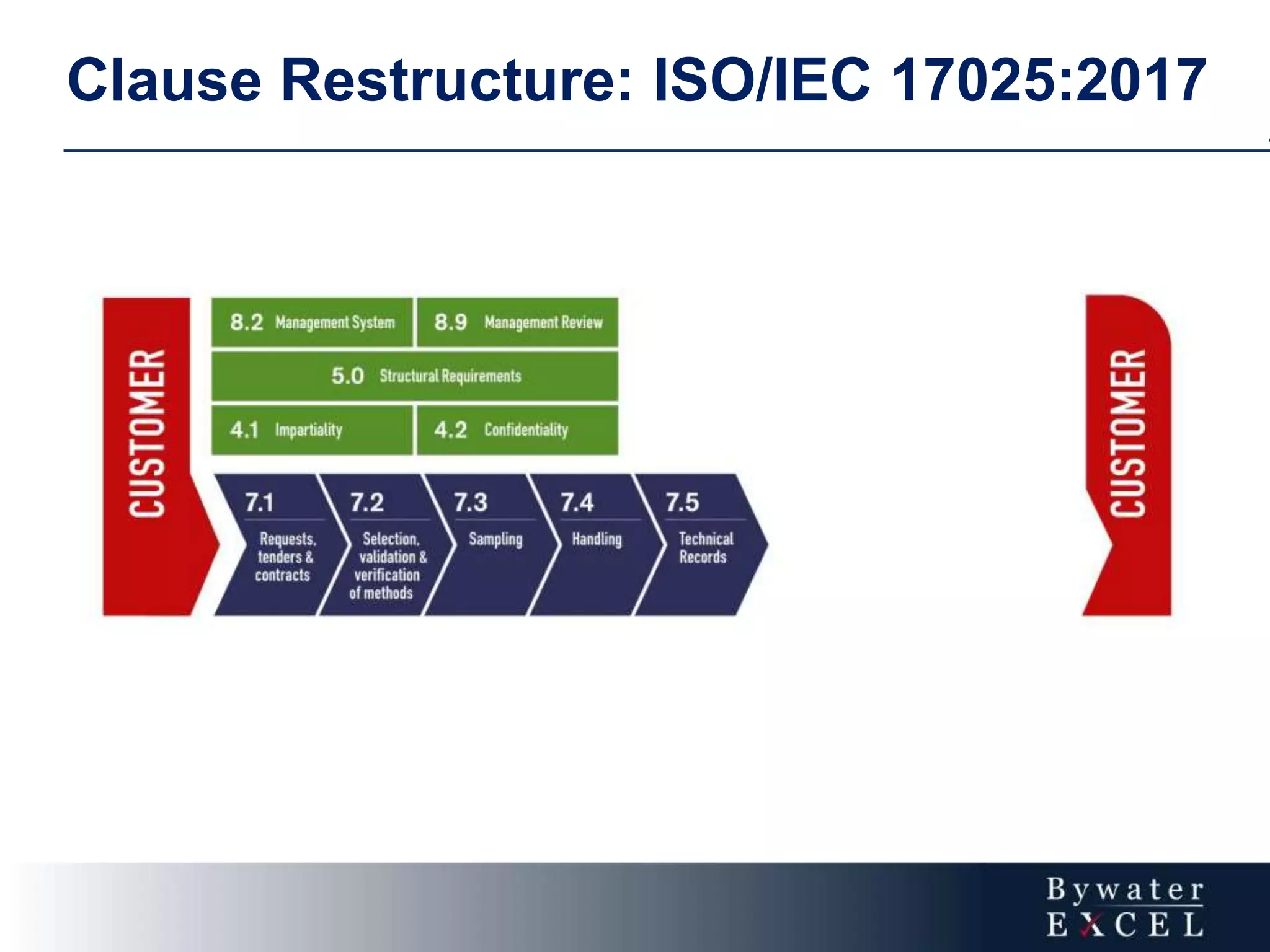
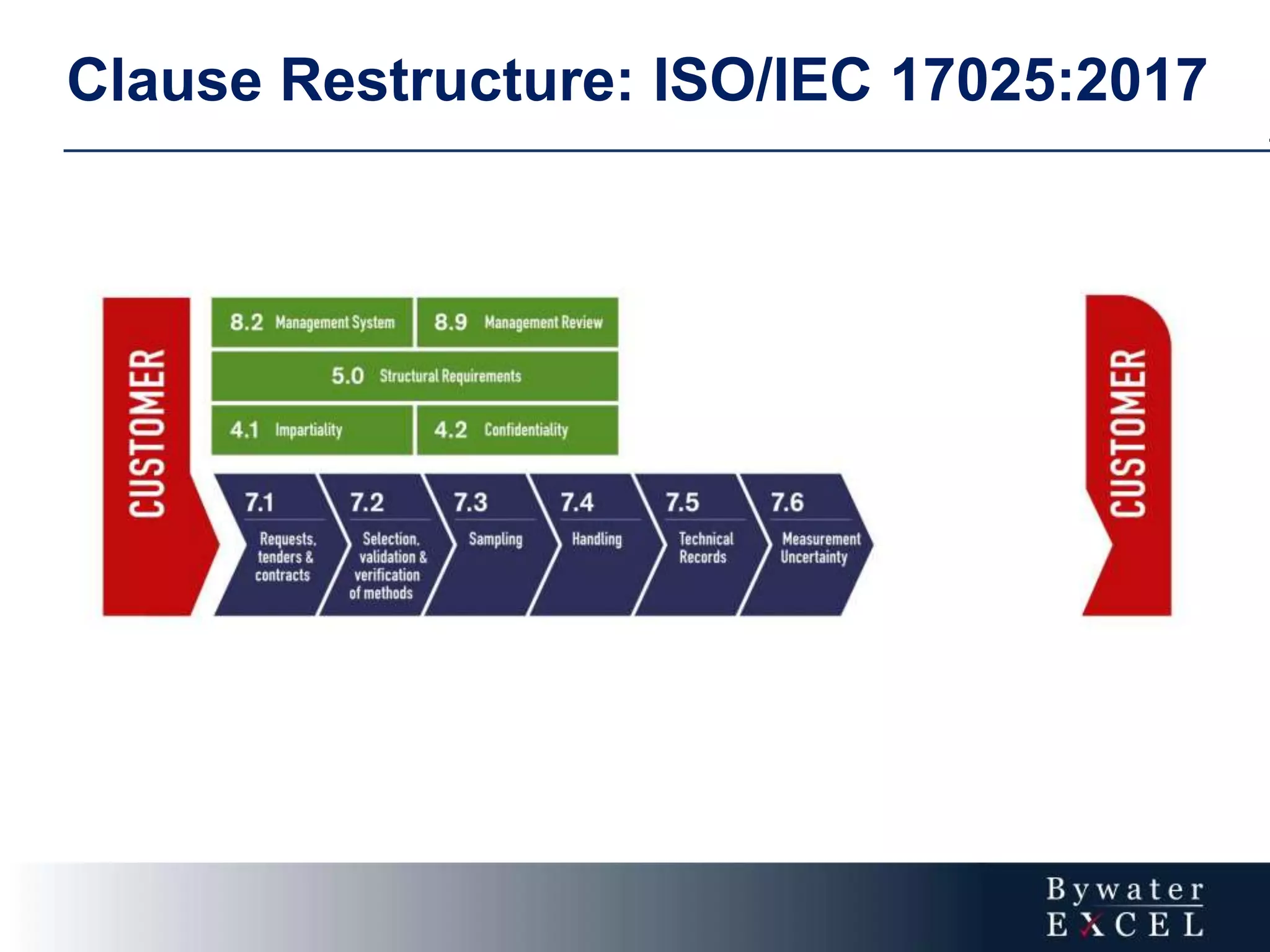
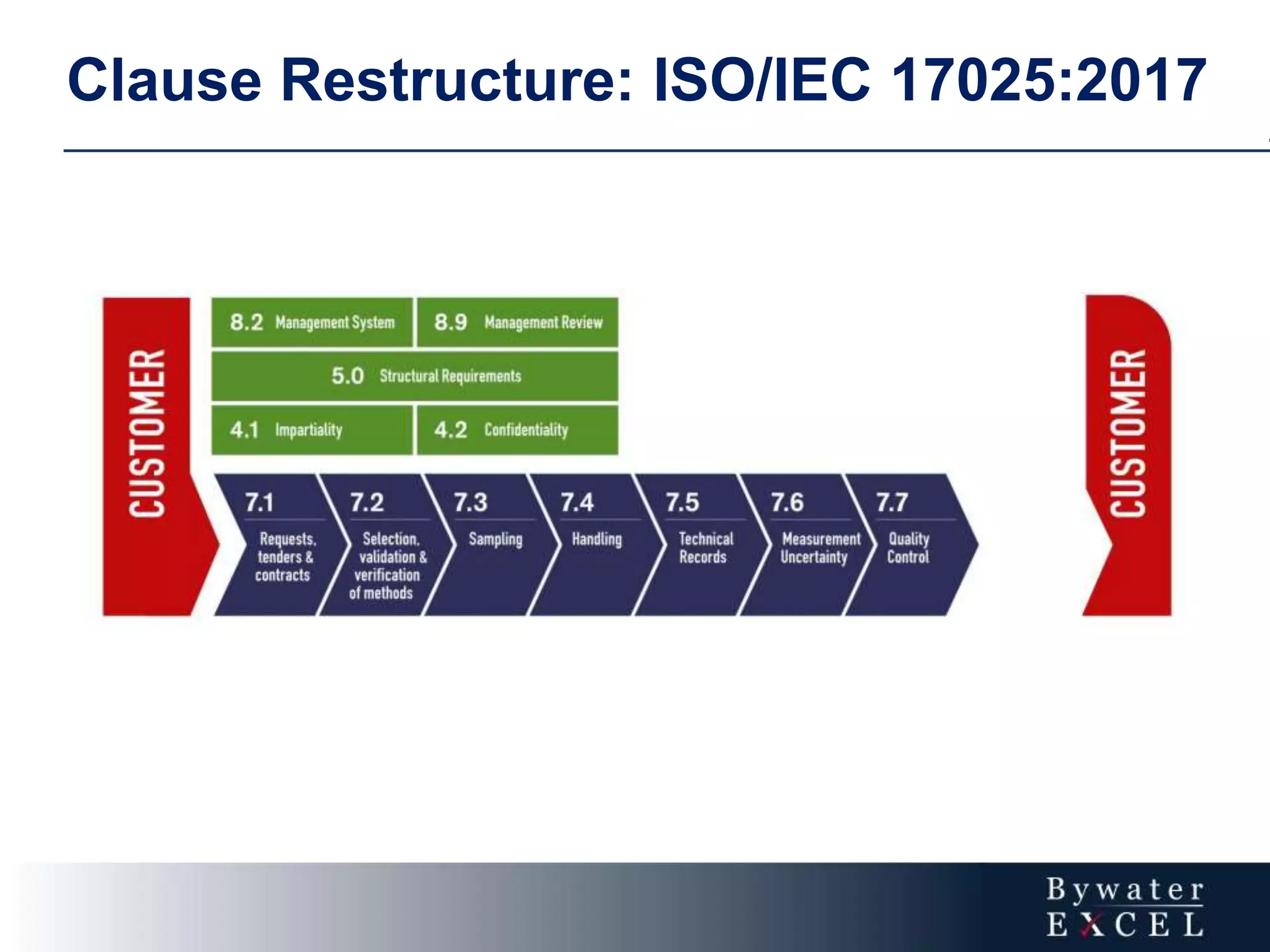
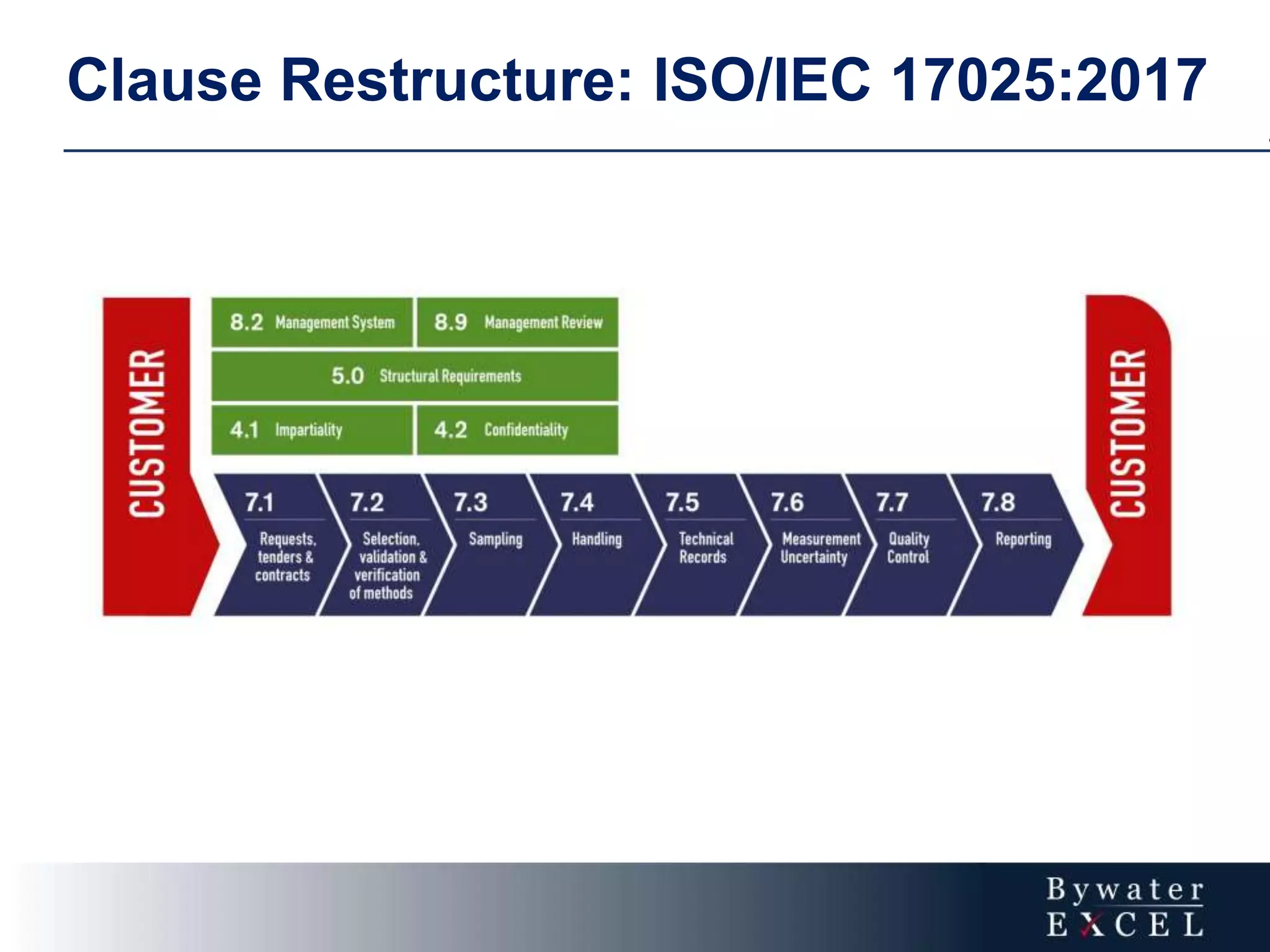
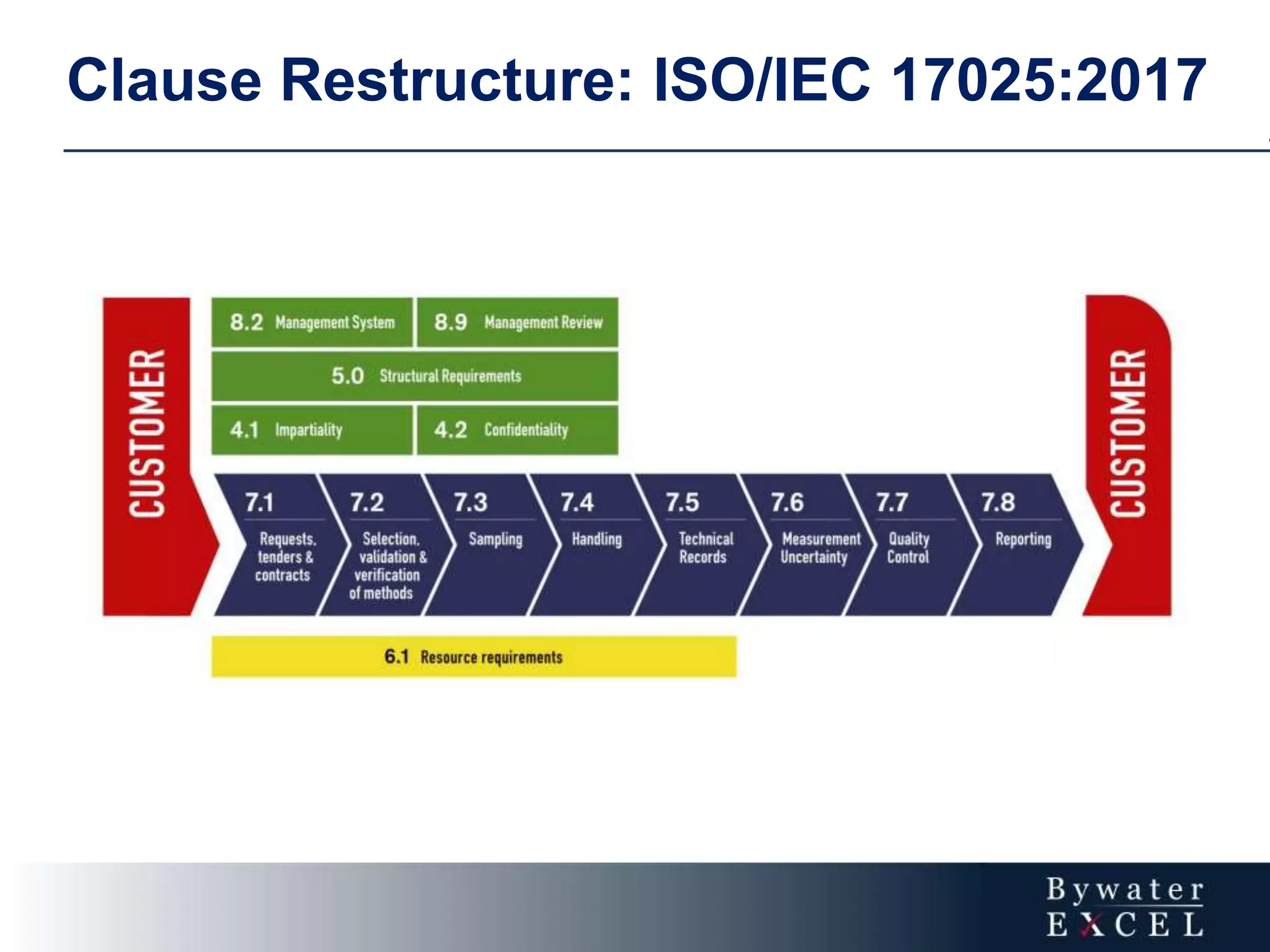
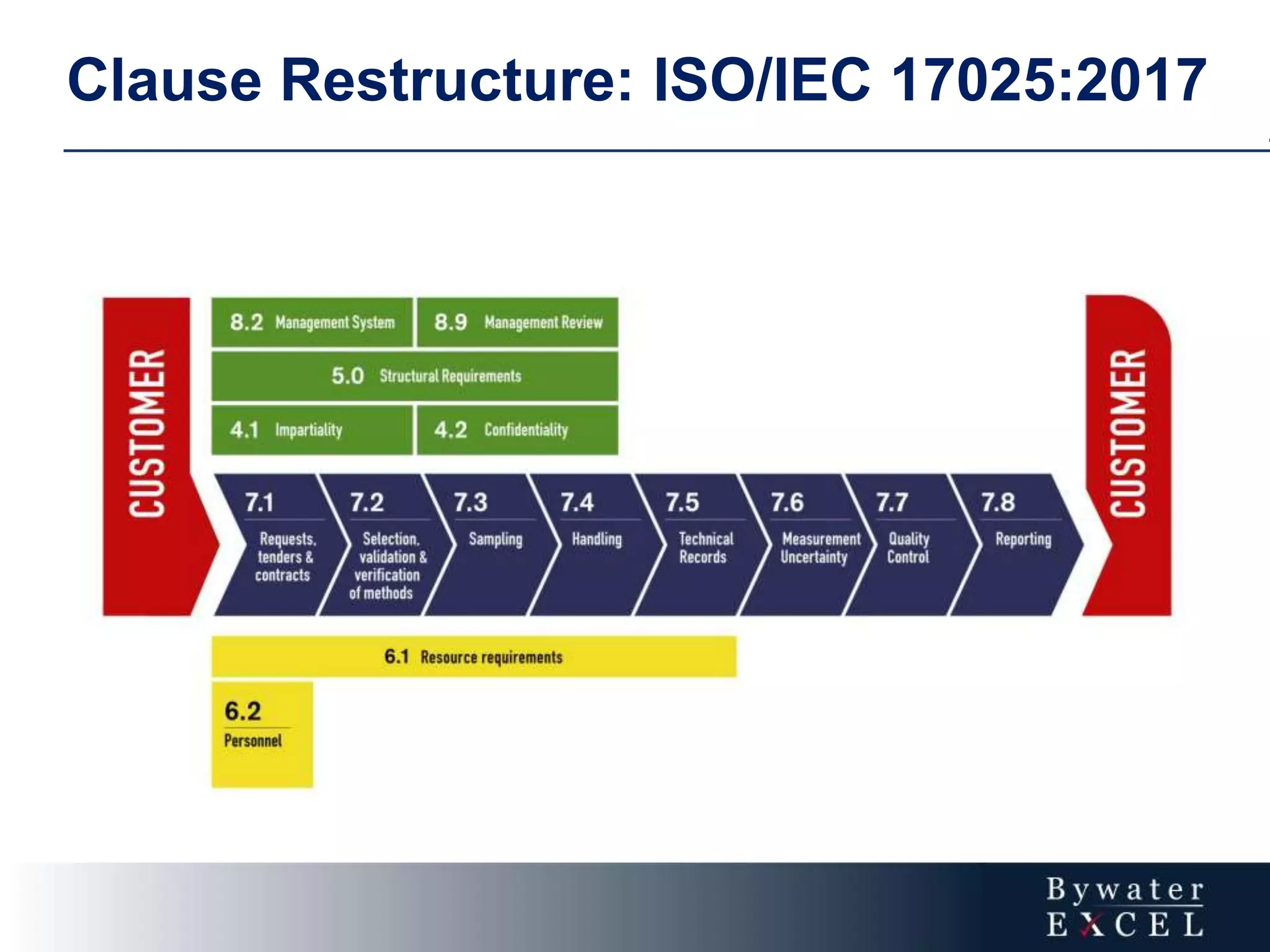
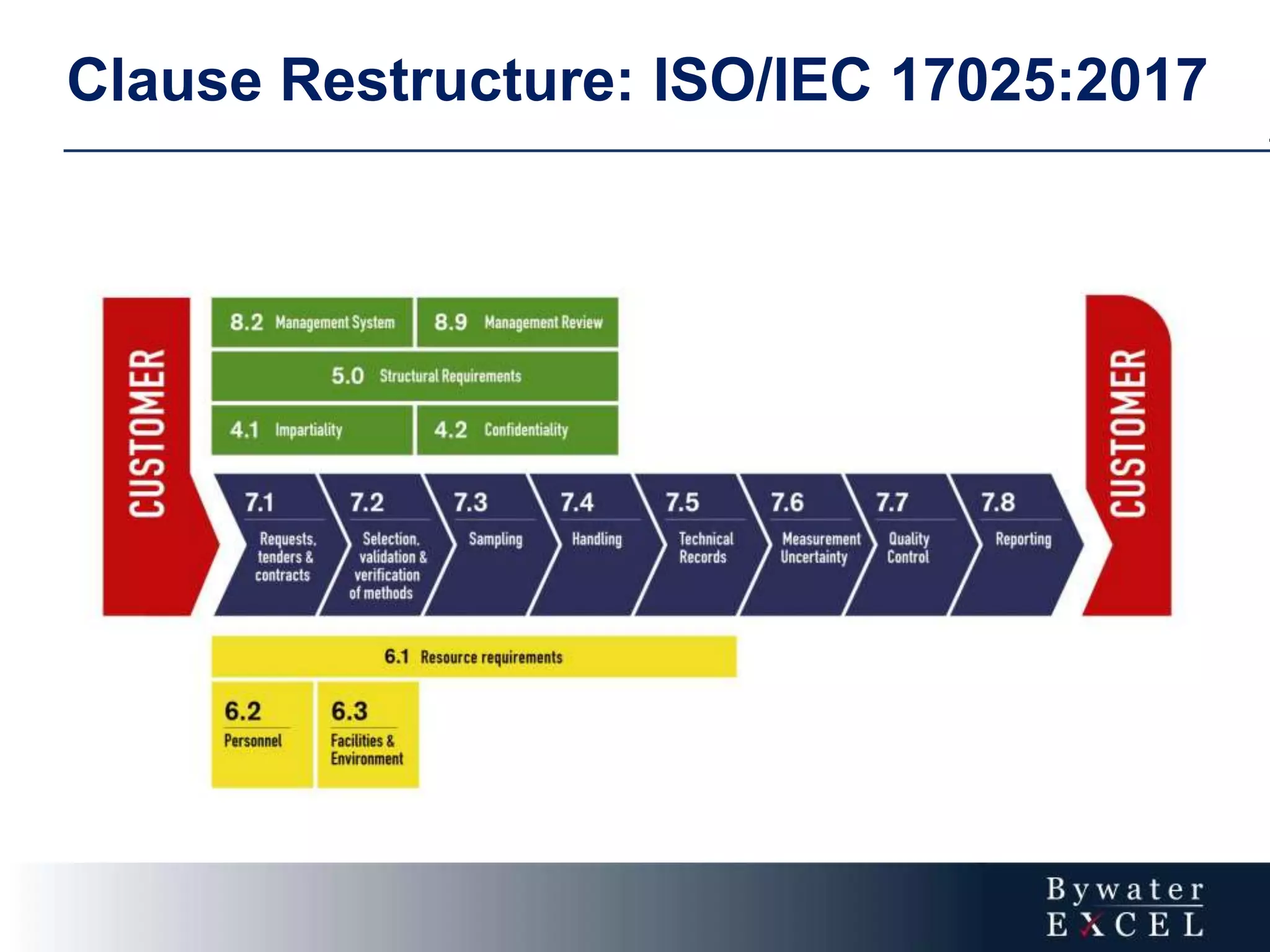
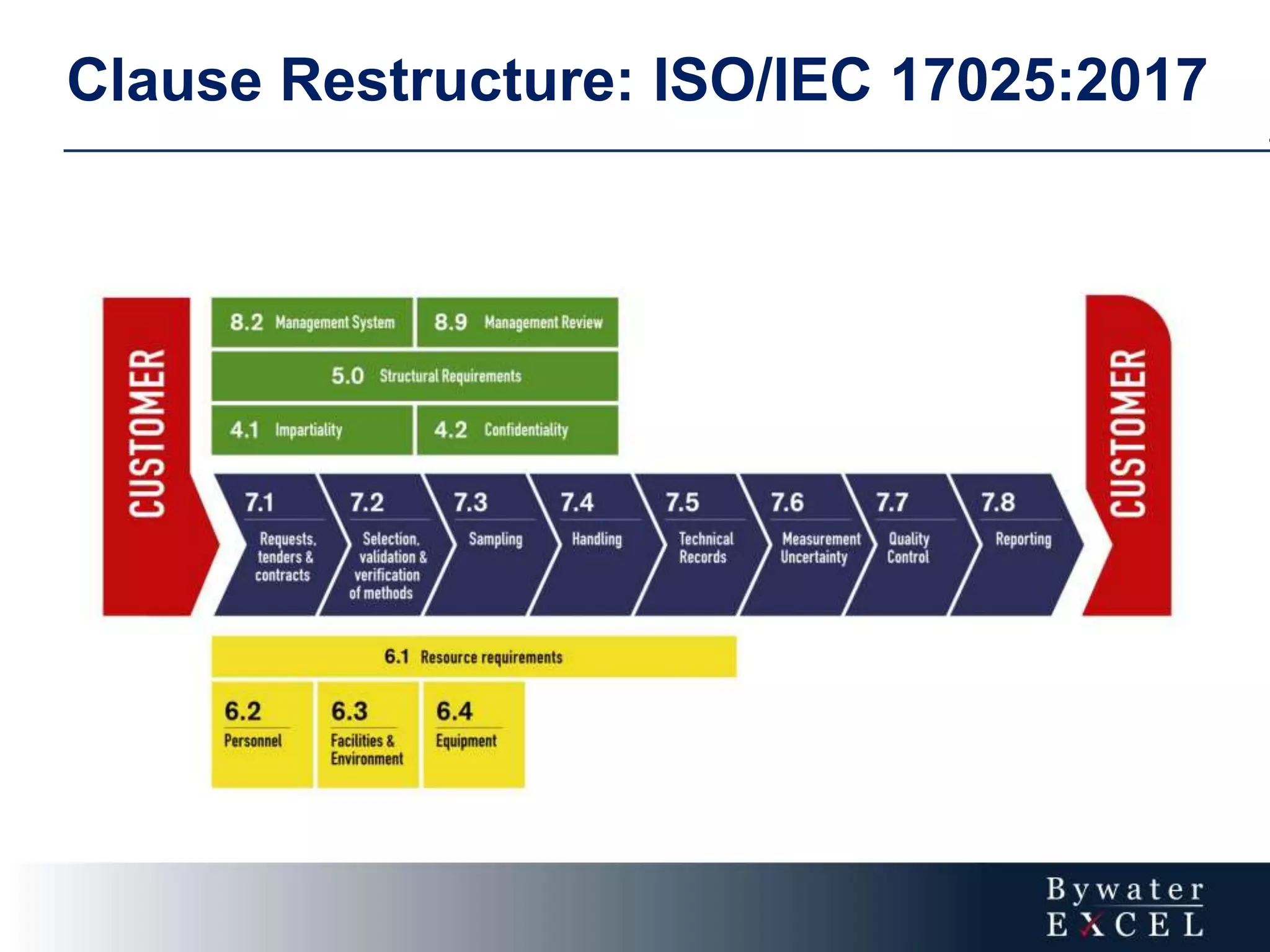
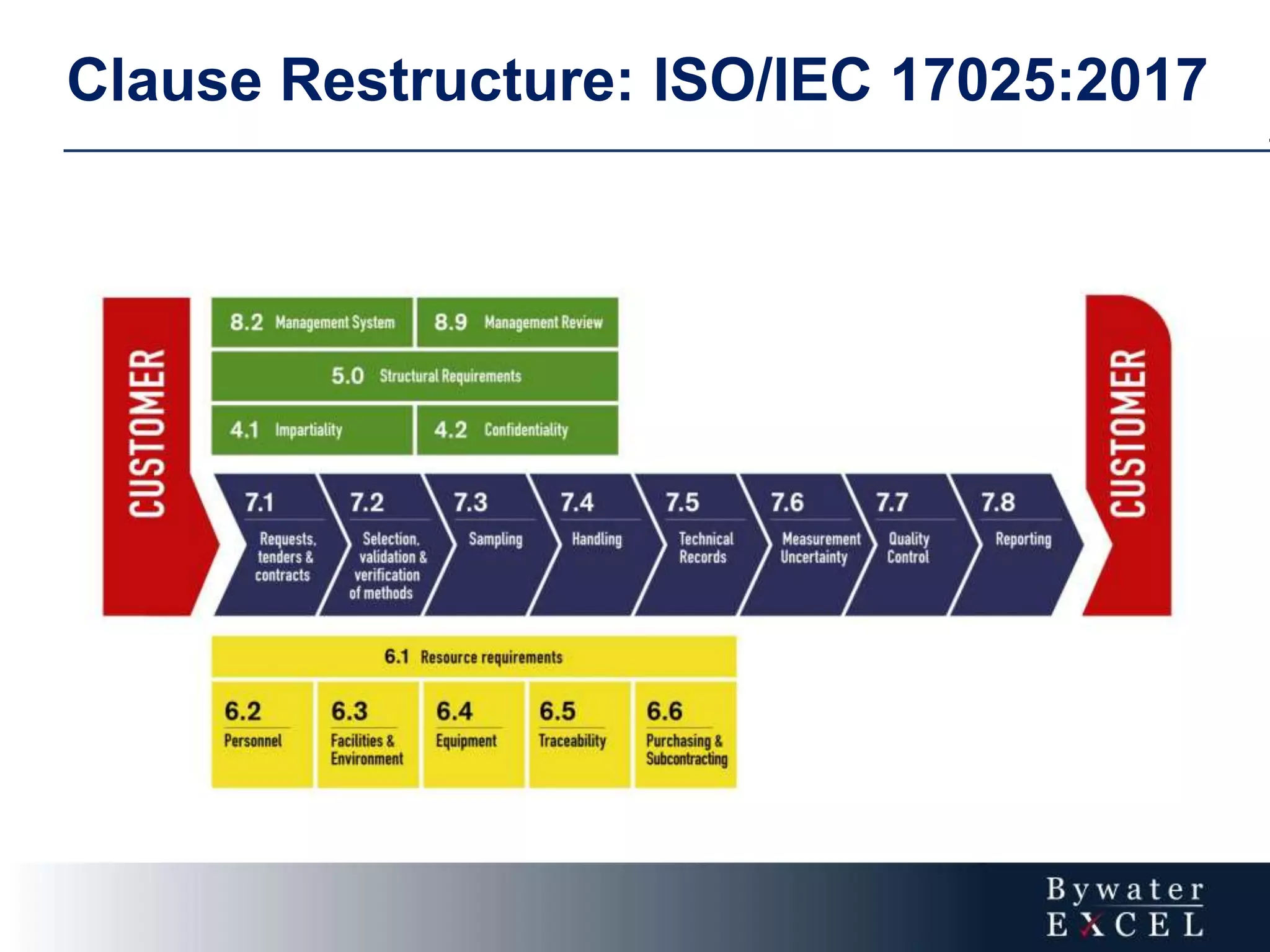
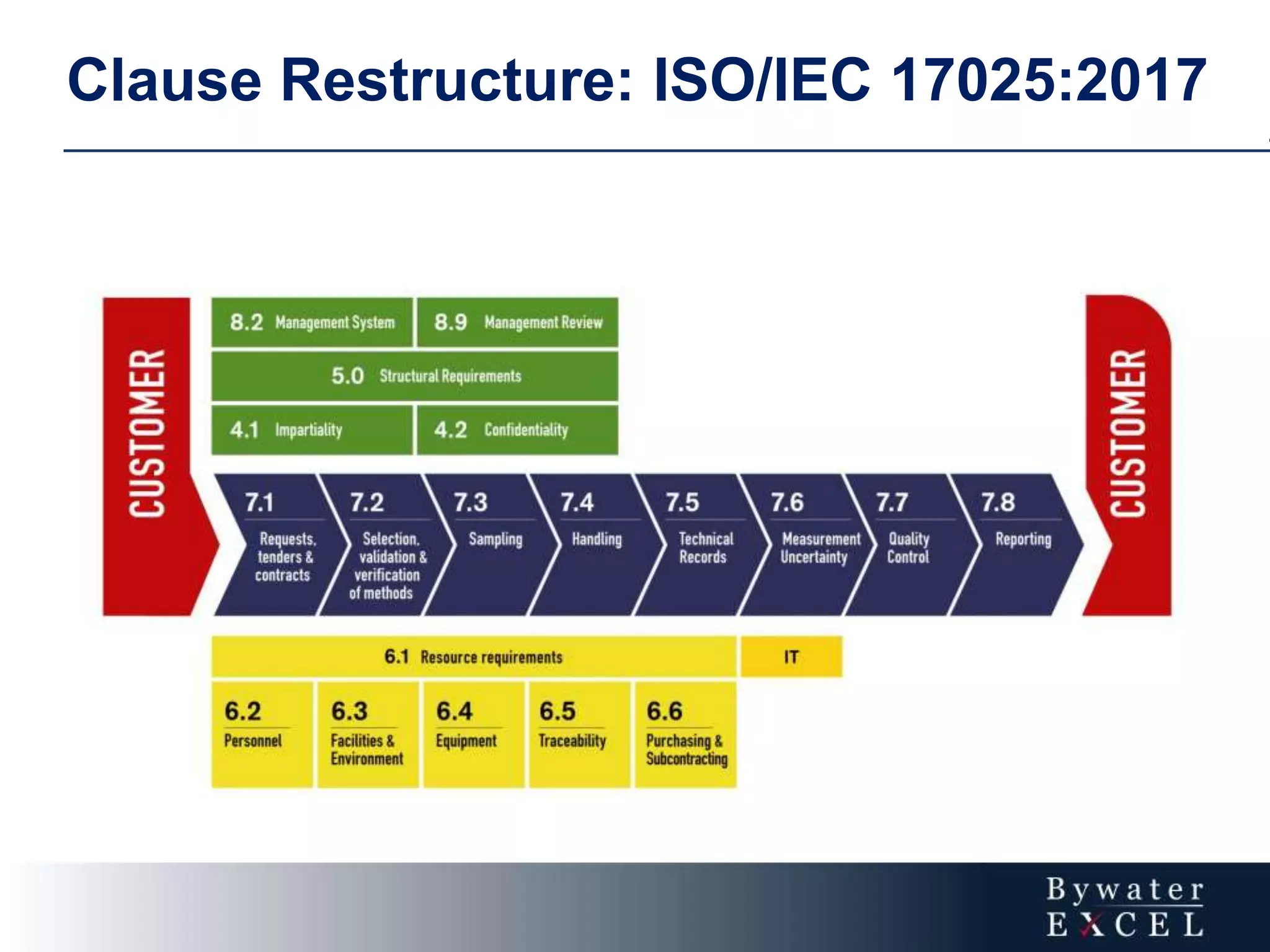
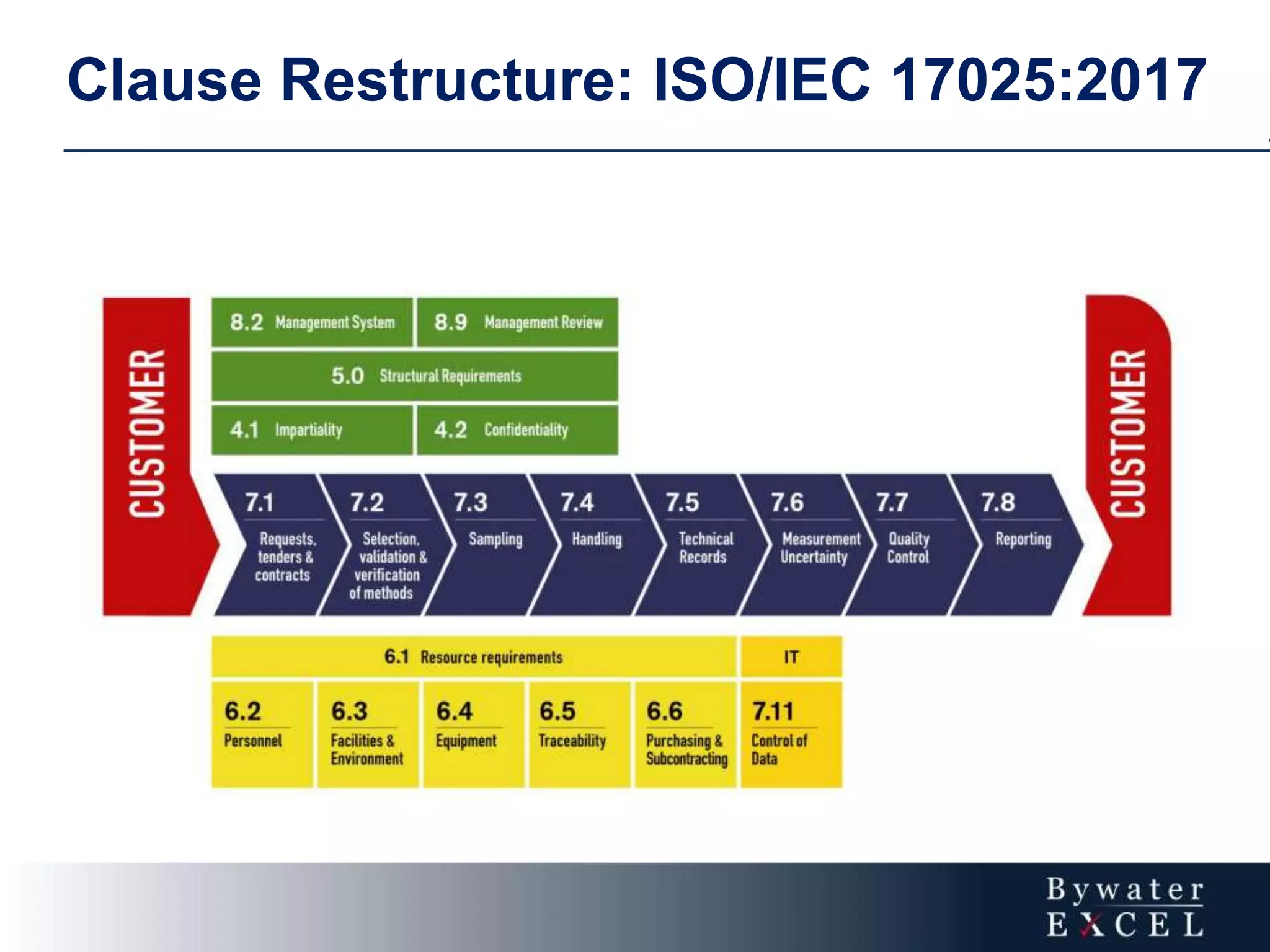
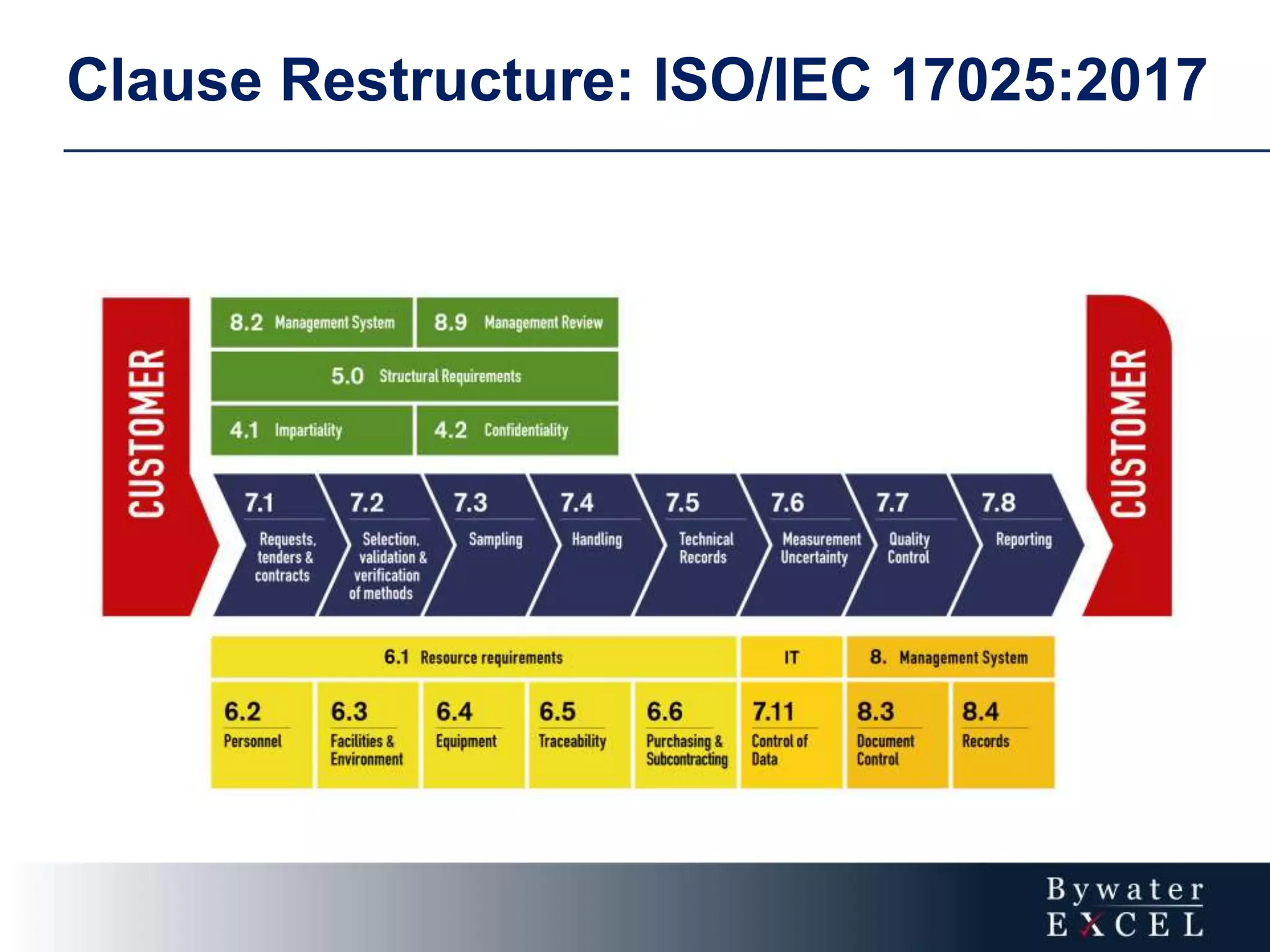
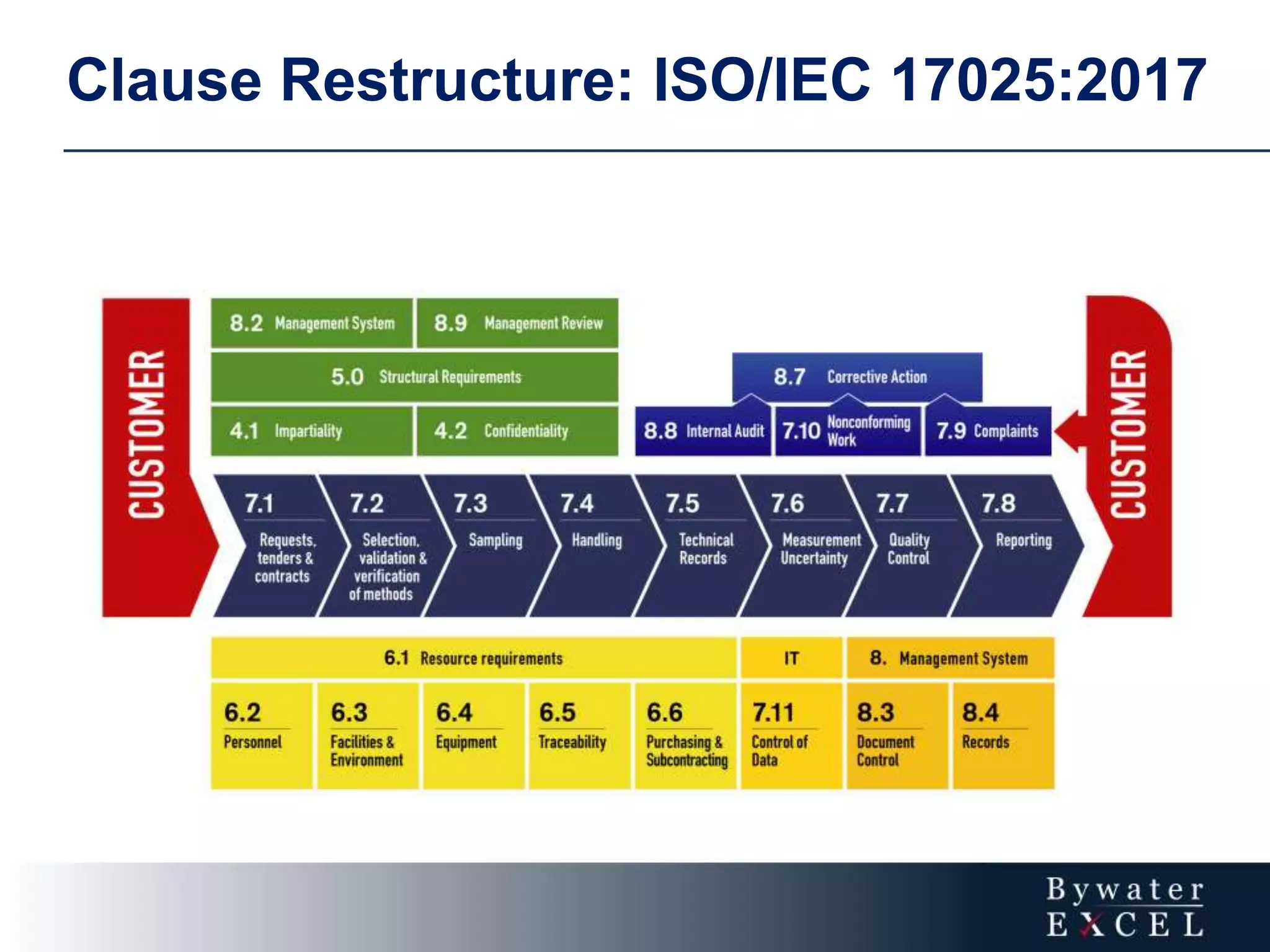
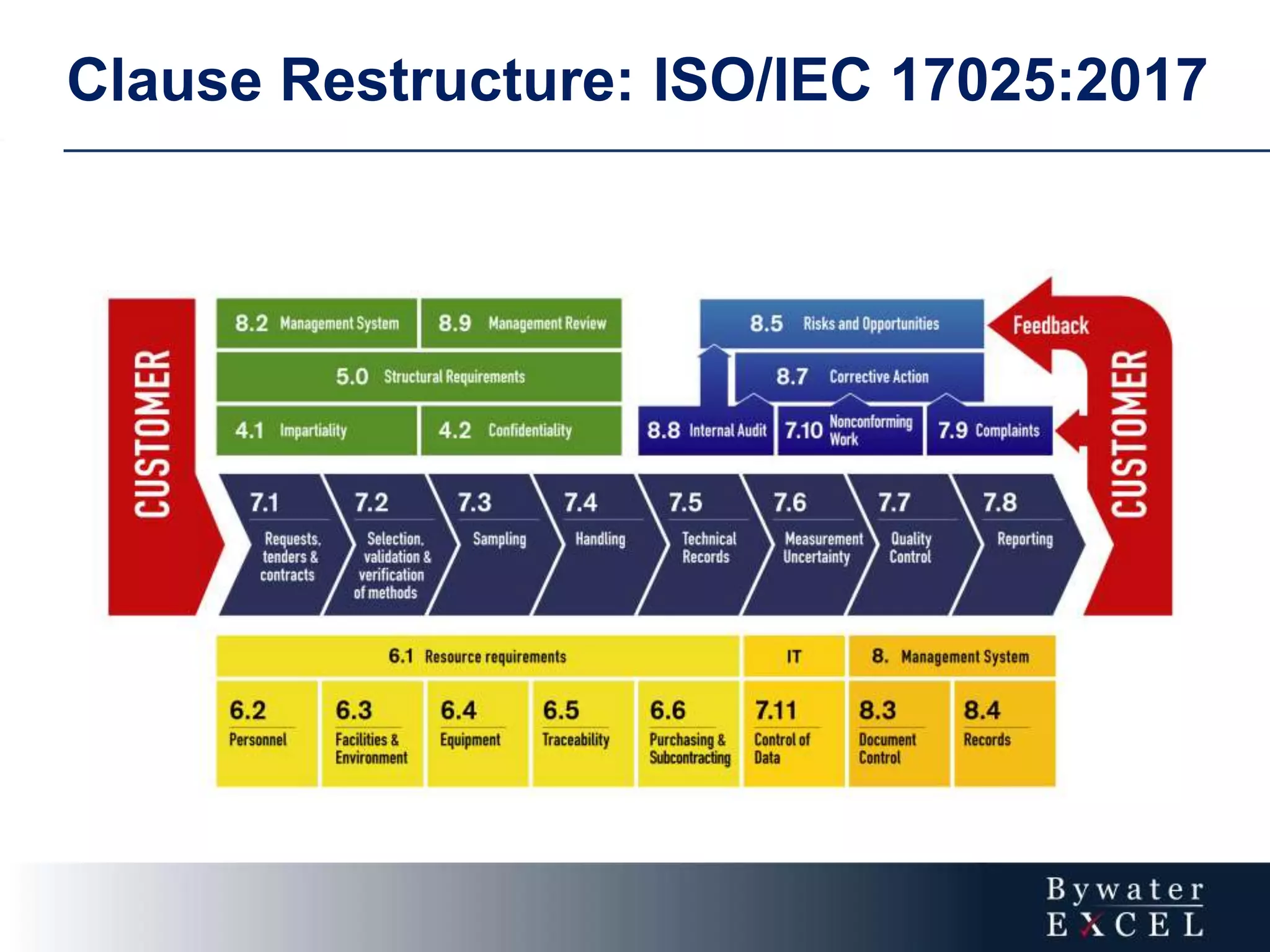
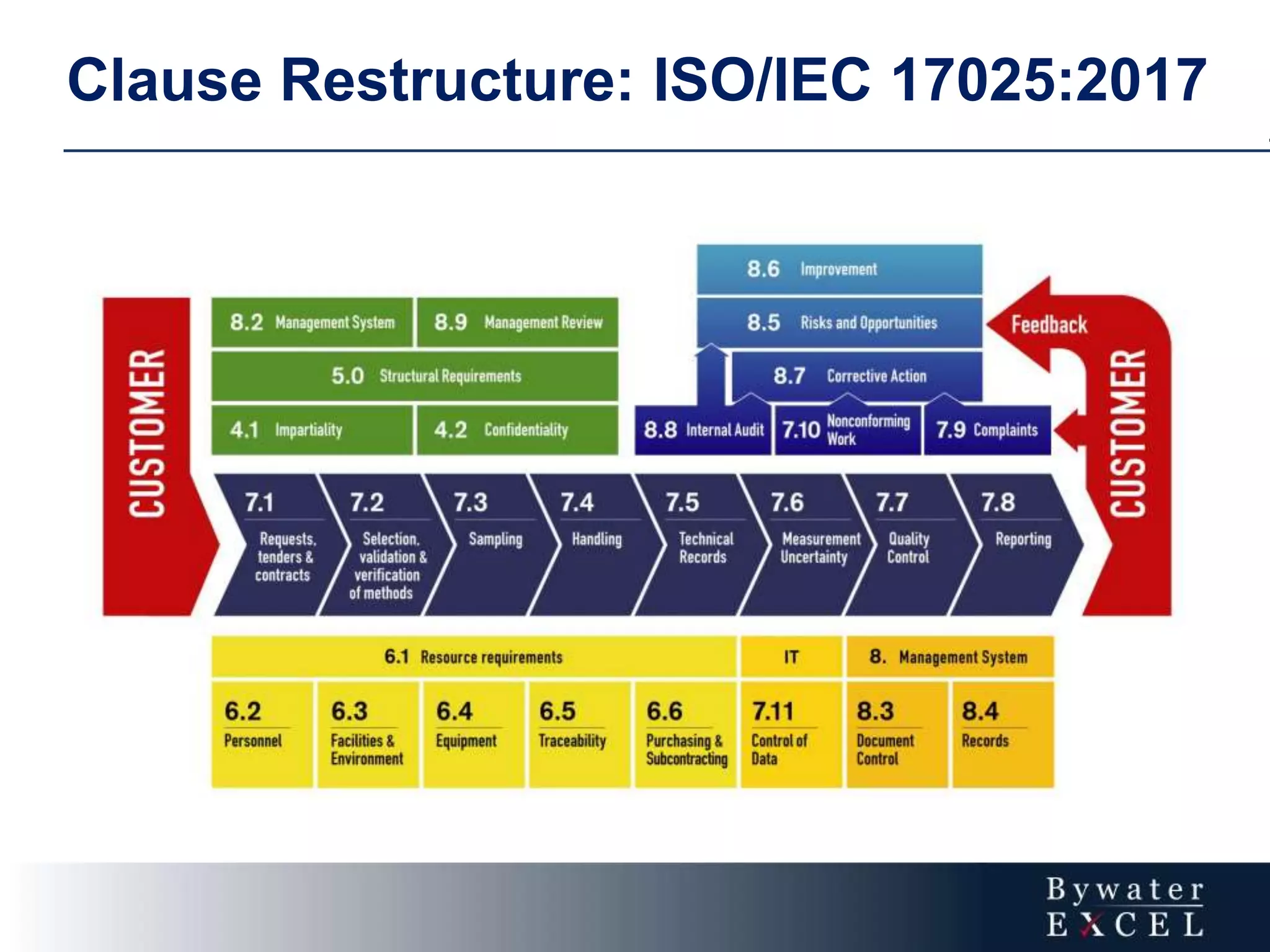
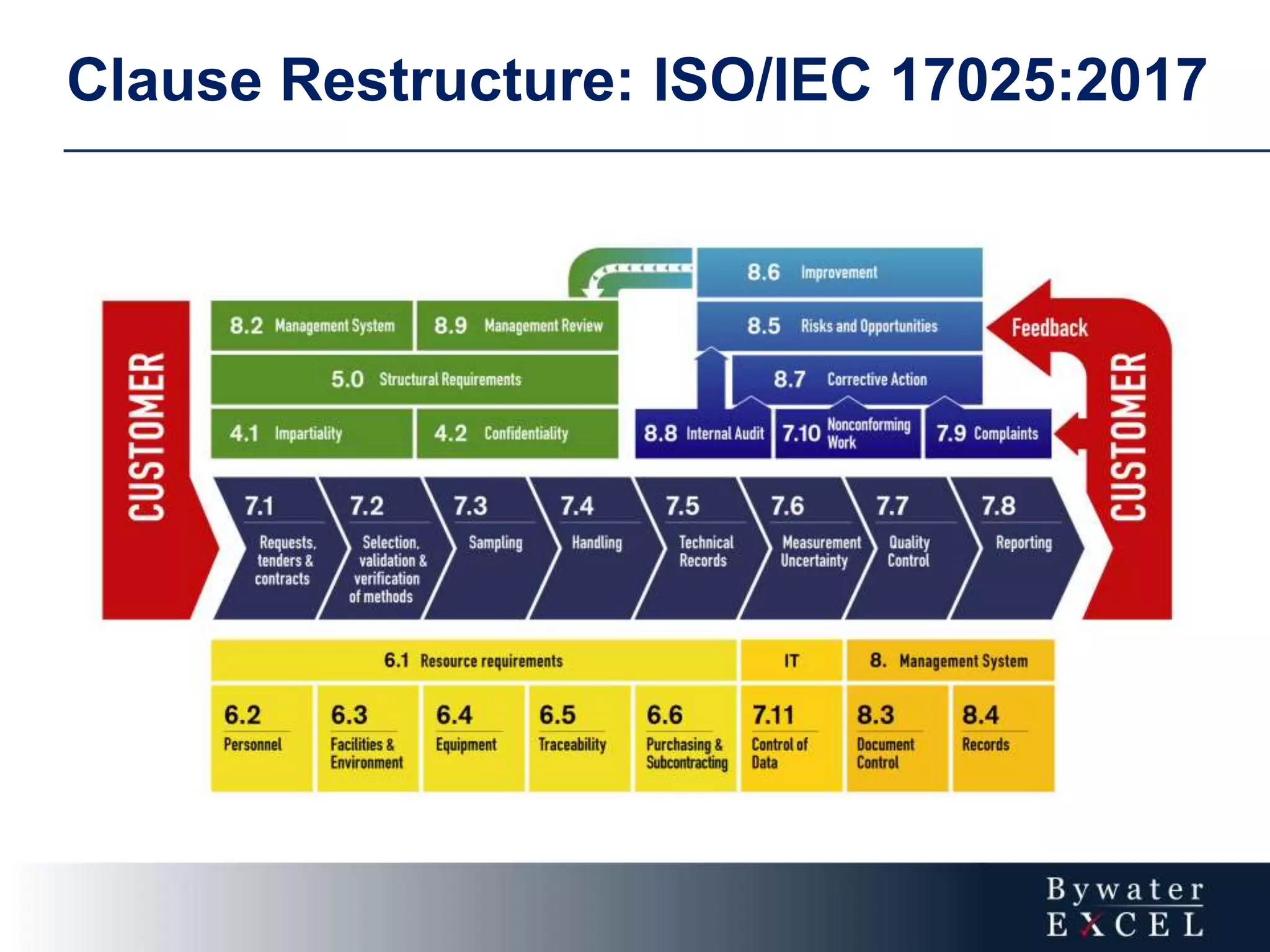
The document discusses changes made to ISO/IEC 17025:2005 in the 2017 update. Key changes include restructuring clause numbers, additional requirements for impartiality, risk assessment, complaints handling, and data management. Laboratories have three years to transition to the new standard. The document also provides information on training courses to help laboratories understand and implement the changes required to comply with ISO/IEC 17025:2017.