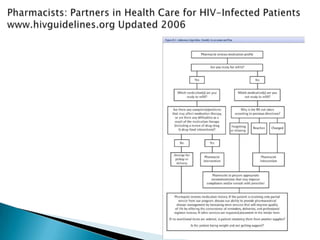
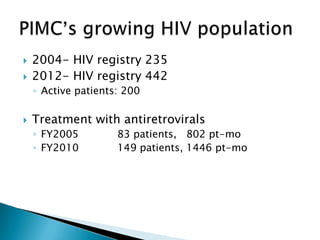
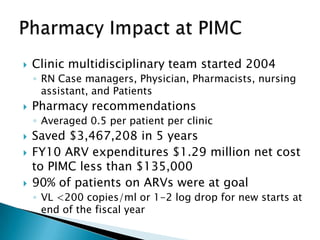
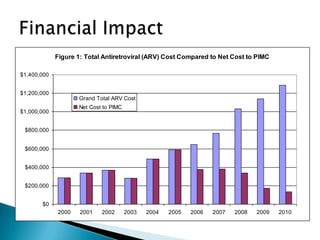
This document discusses various roles that pharmacists can take in HIV management, screening, and care. It describes how pharmacists can be involved in lead screening, care management, and providing post-exposure prophylaxis (PEP). The document also outlines funding opportunities for pharmacists and facilities to expand their involvement in HIV initiatives.