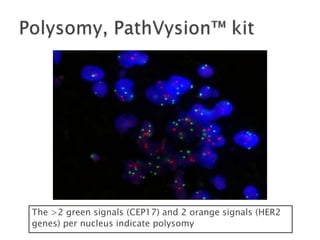
This document discusses HER-2 testing in breast cancer and updates guidelines. It notes that HER-2 positive breast cancer is a heterogeneous disease that can be targeted by specific drugs like Herceptin. Accurate HER-2 testing is important to identify patients who will benefit from Herceptin therapy, as false results could deny patients effective treatment or cause patients to receive unnecessary treatment. The document reviews HER-2 testing methods and provides guidelines on confirming HER-2 status in ambiguous cases.