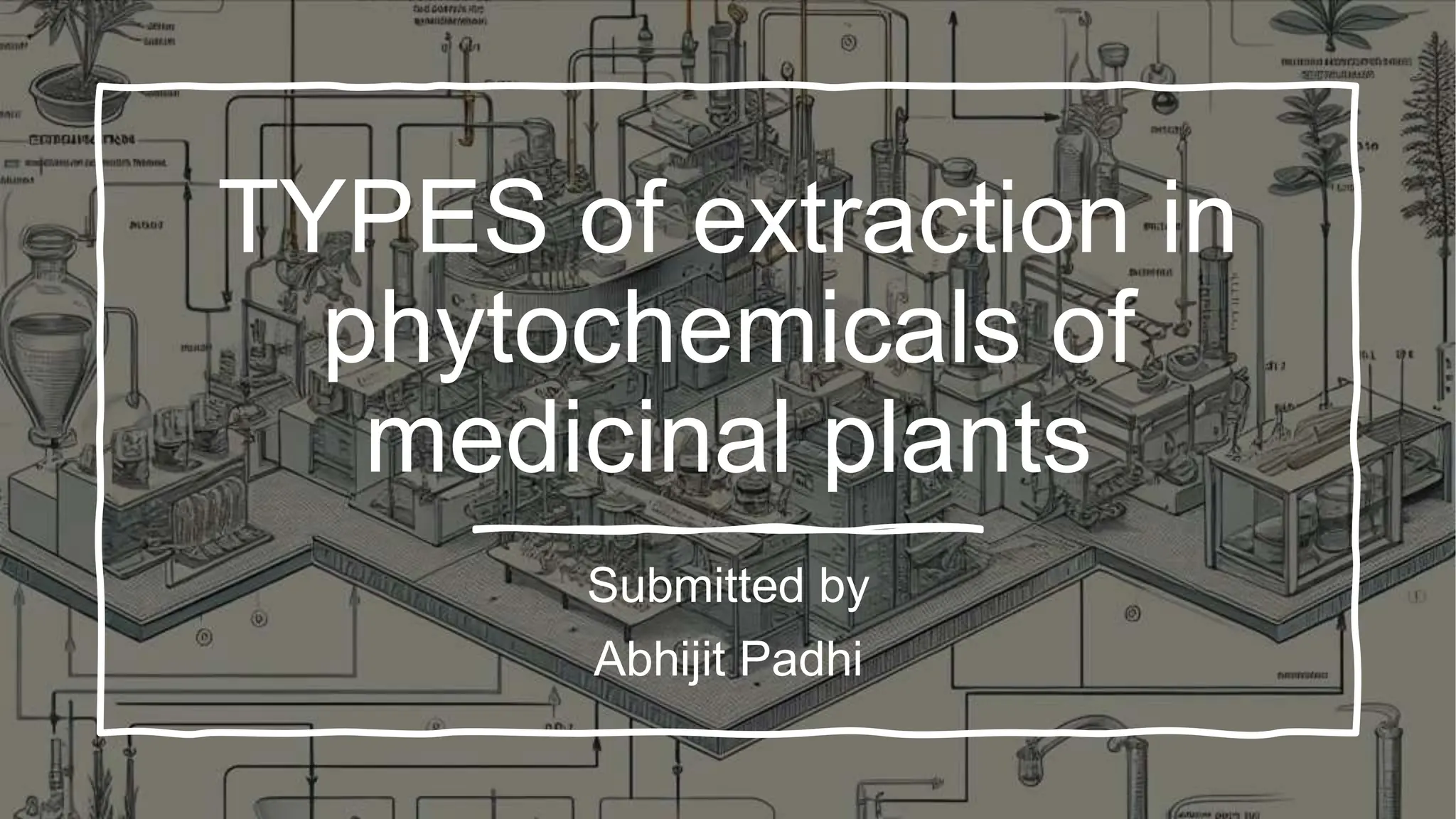
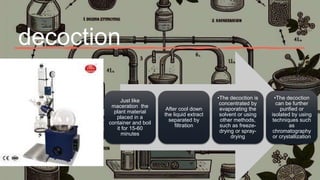
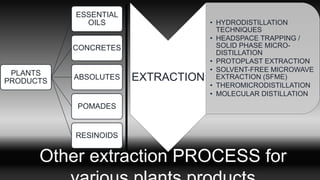
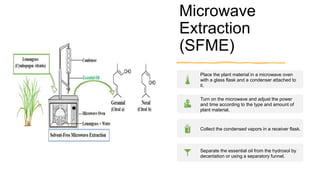
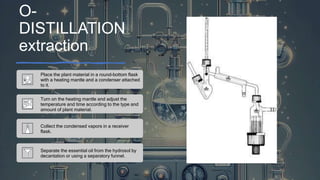
This document discusses various techniques for extracting phytochemicals from medicinal plants, including maceration, infusion, percolation, digestion, decoction, hot continuous extraction, aqueous-alcoholic extraction, counter-current extraction, microwave-assisted extraction, and ultra-sound extraction. It provides detailed step-by-step explanations of each extraction technique. The goal of extraction is to separate medicinal active compounds from plant materials using solvents and standard procedures.