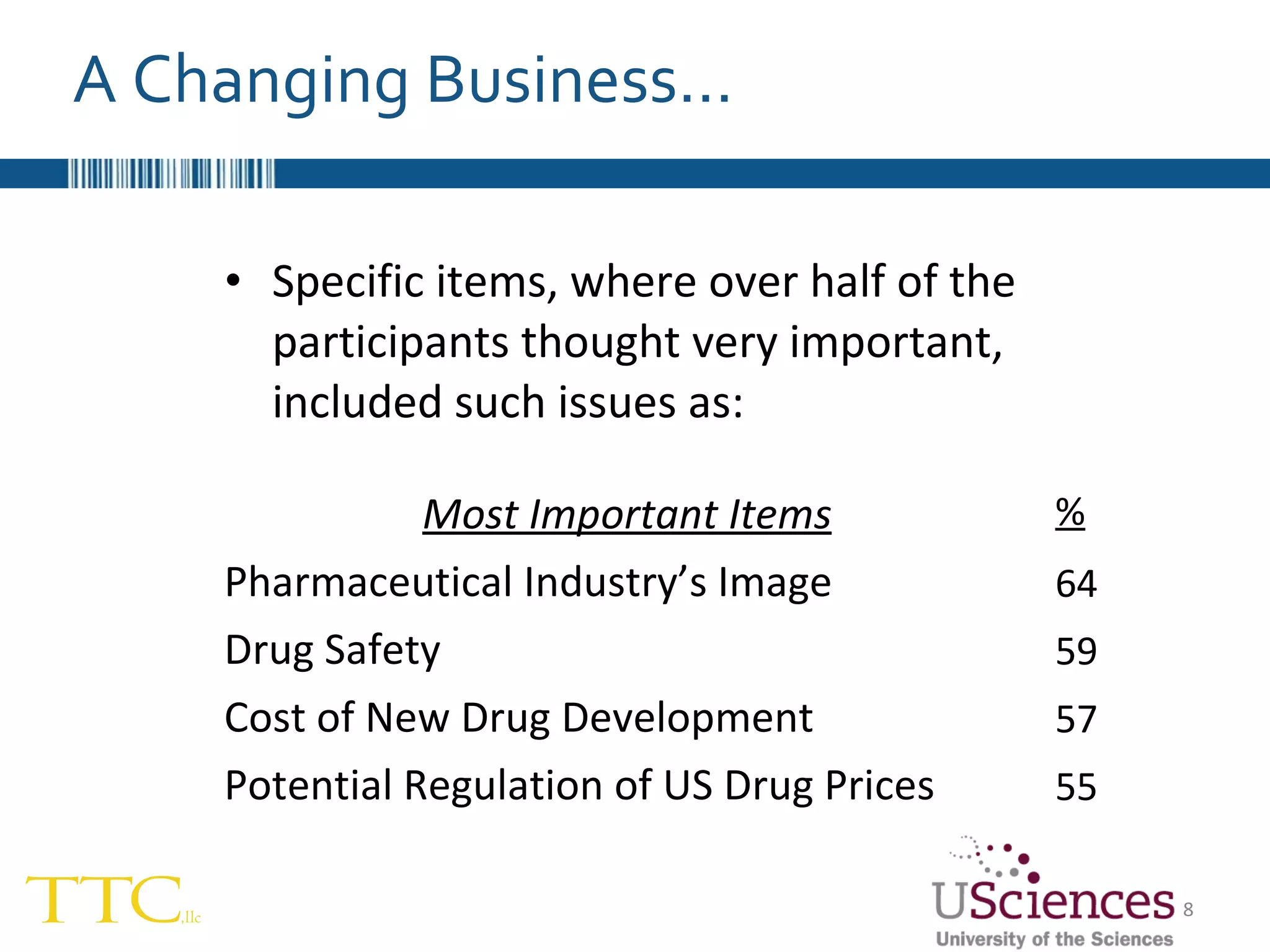
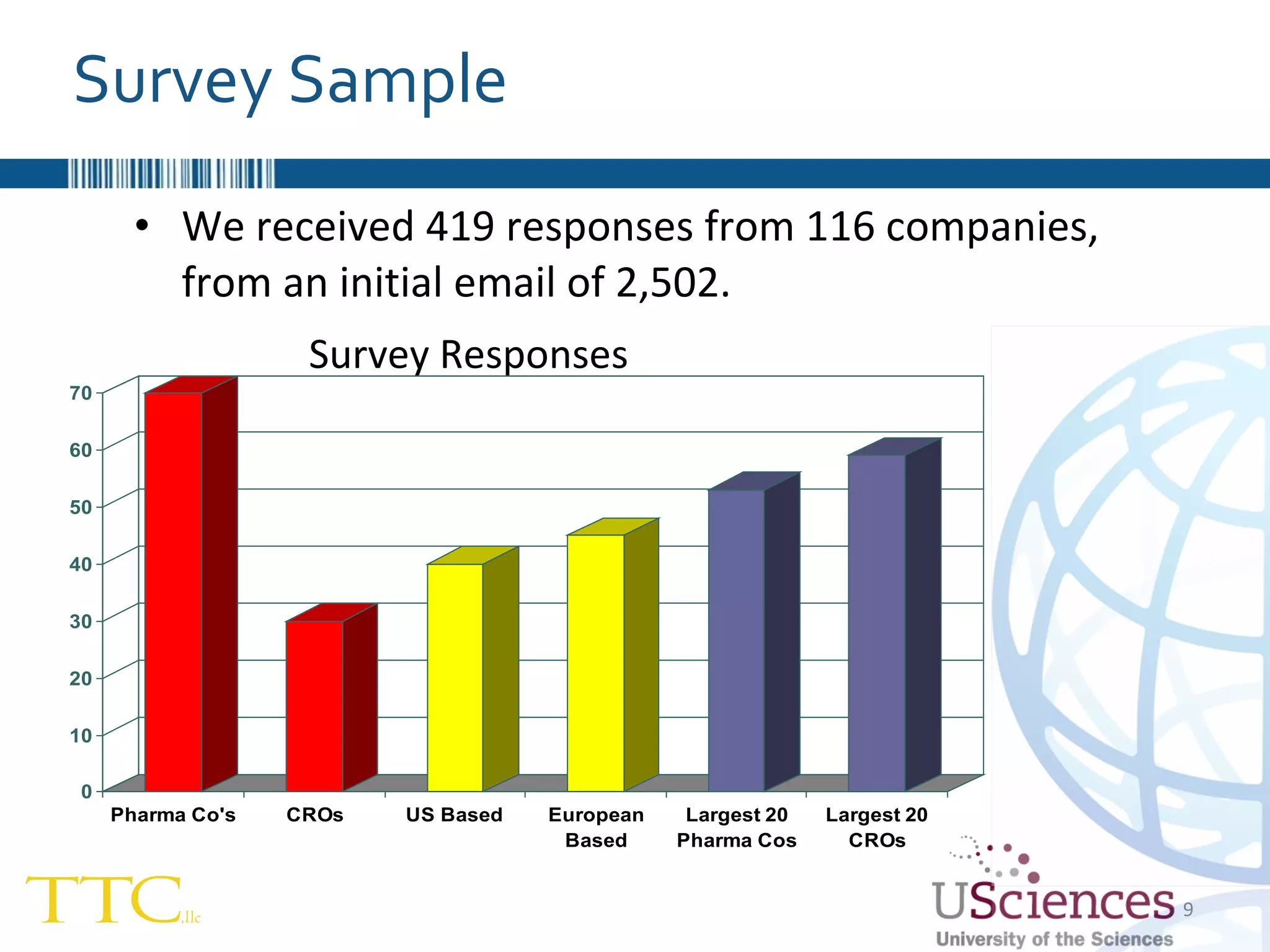
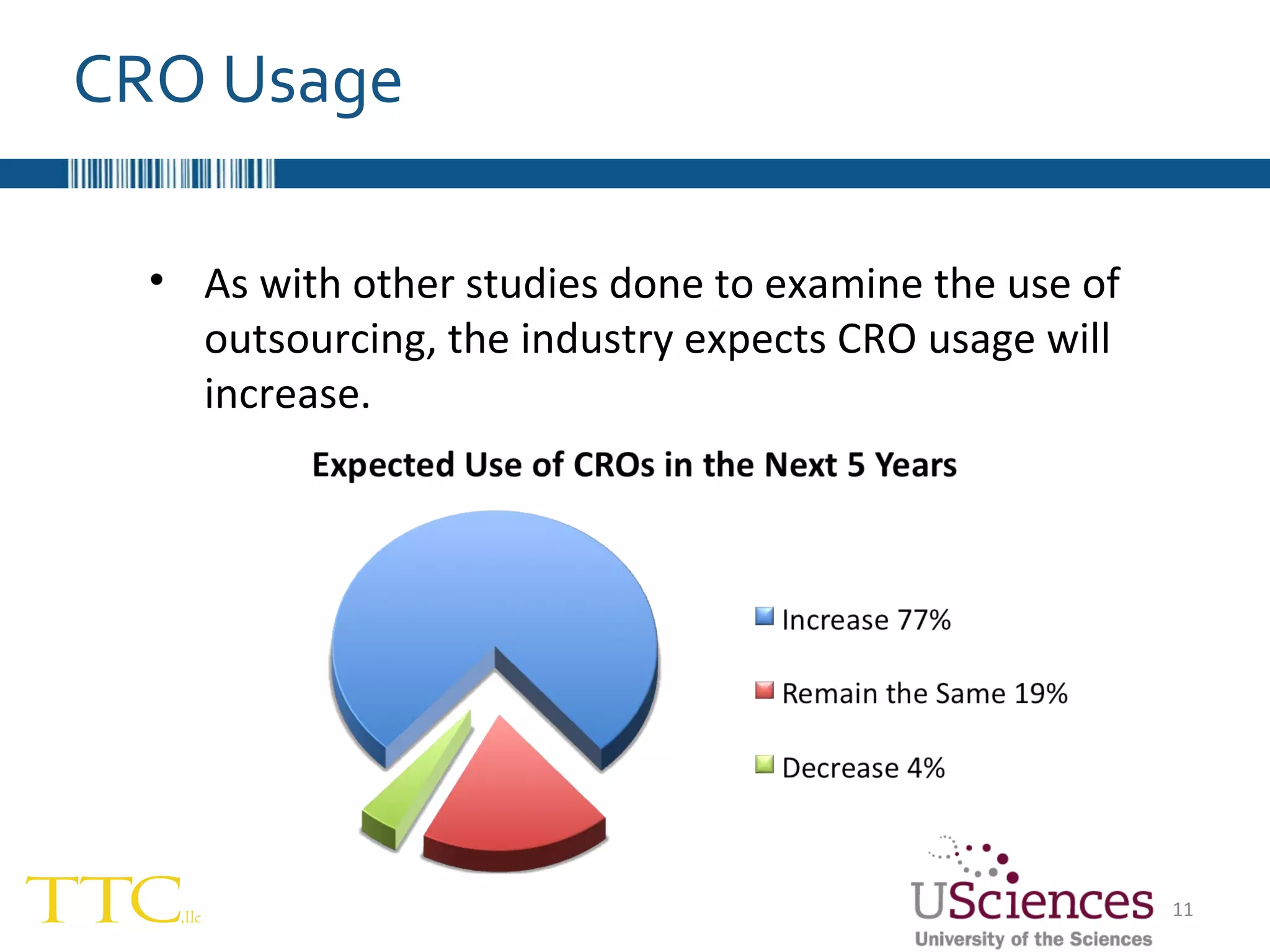
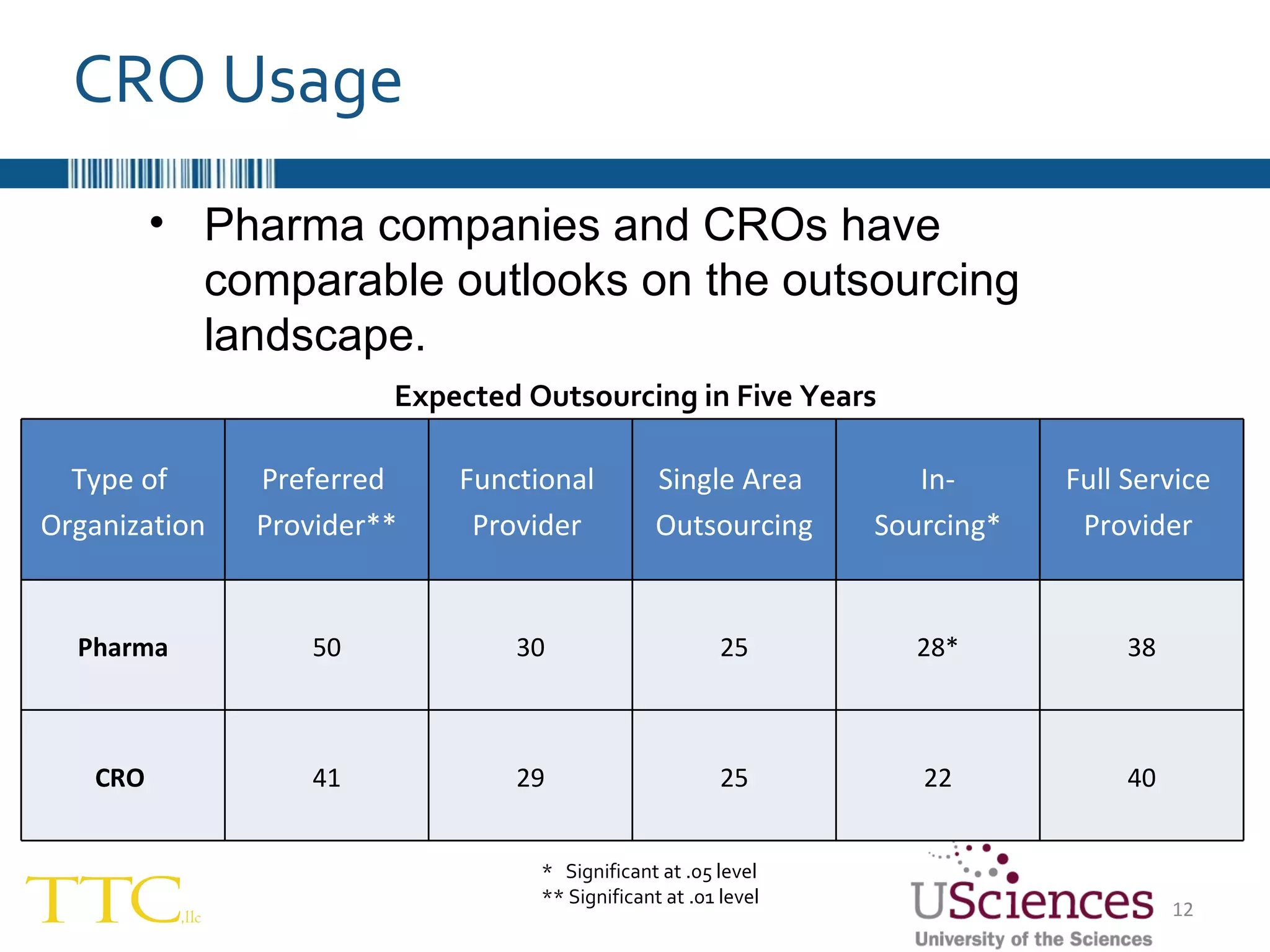
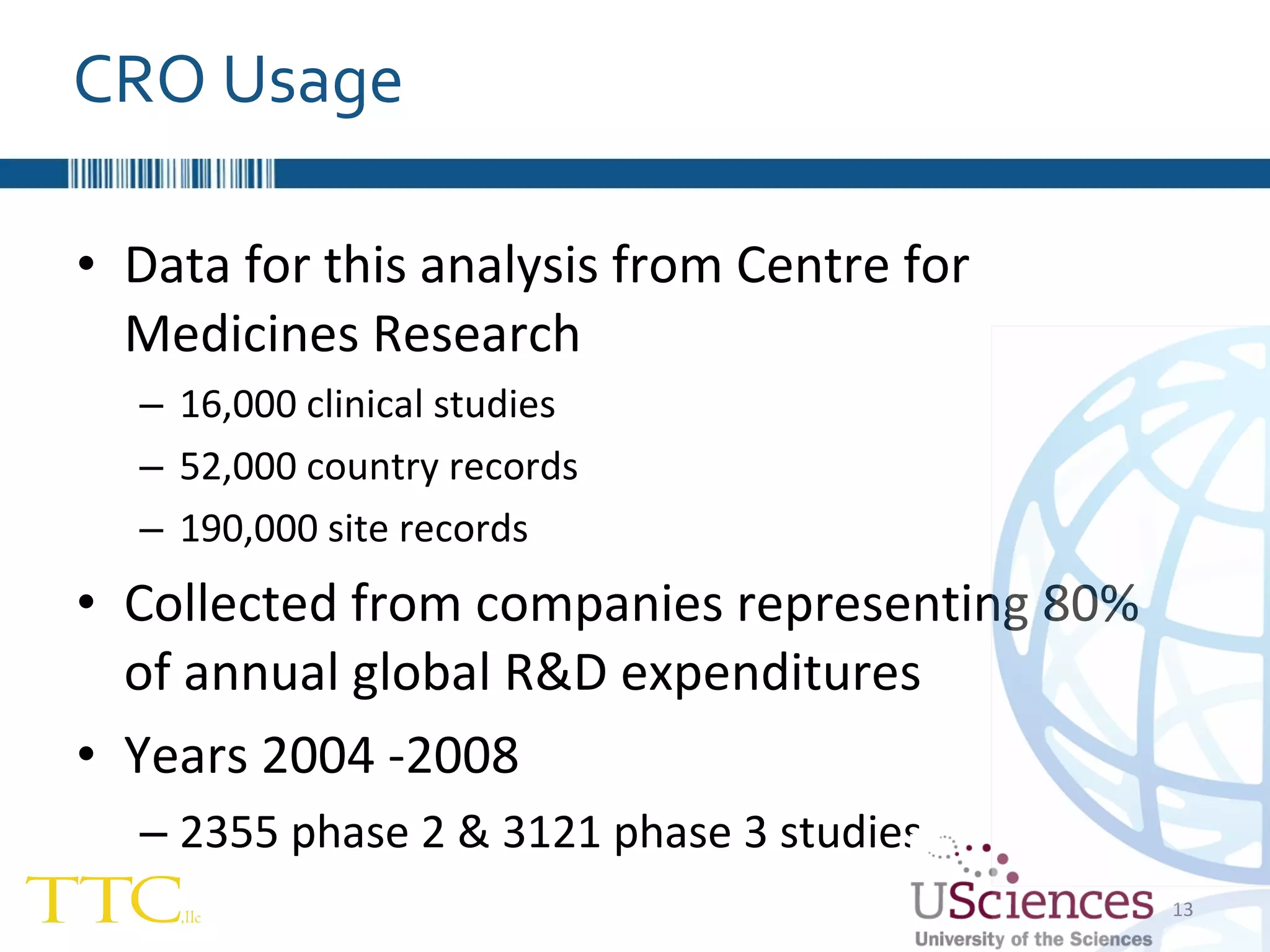
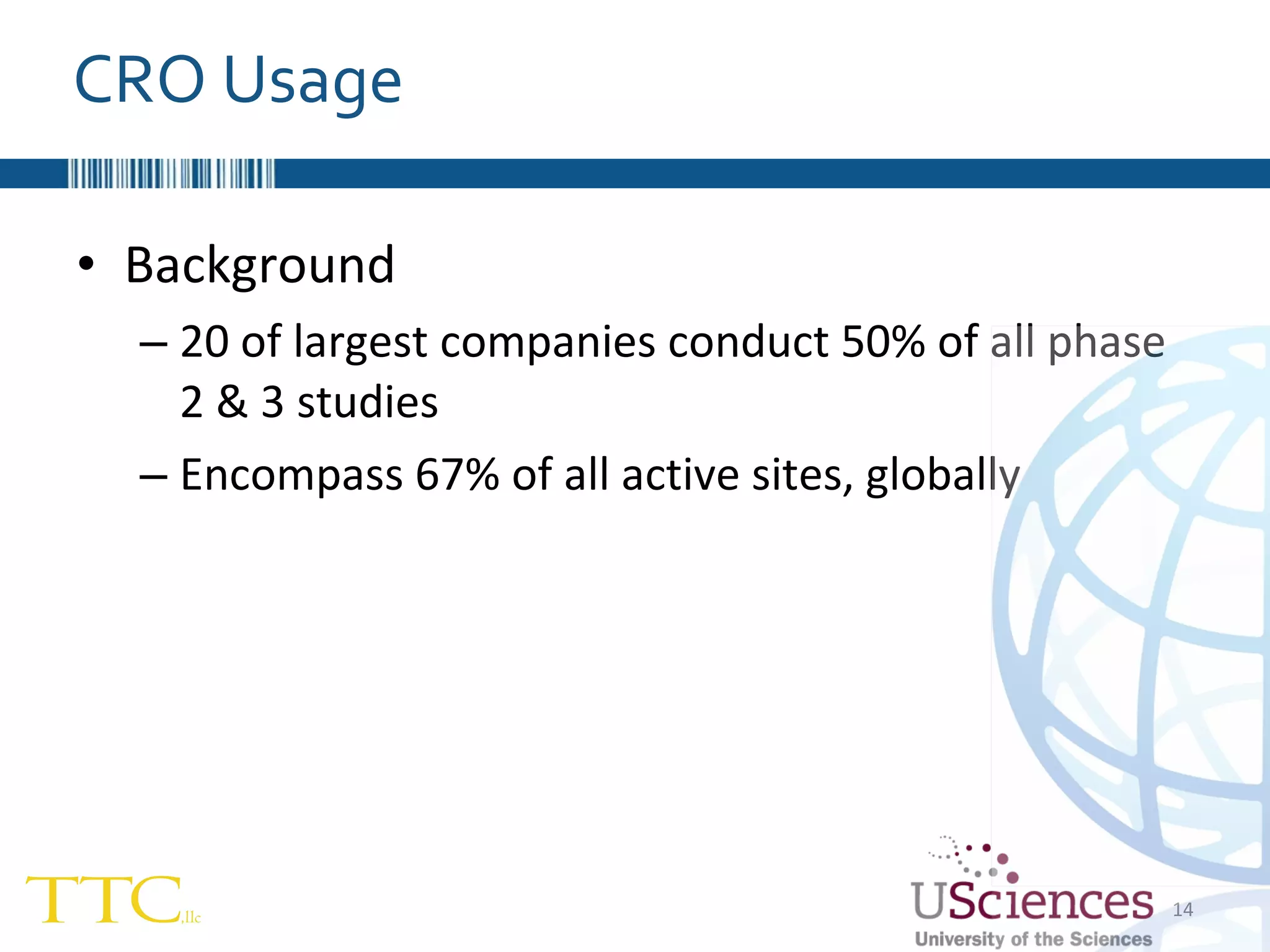
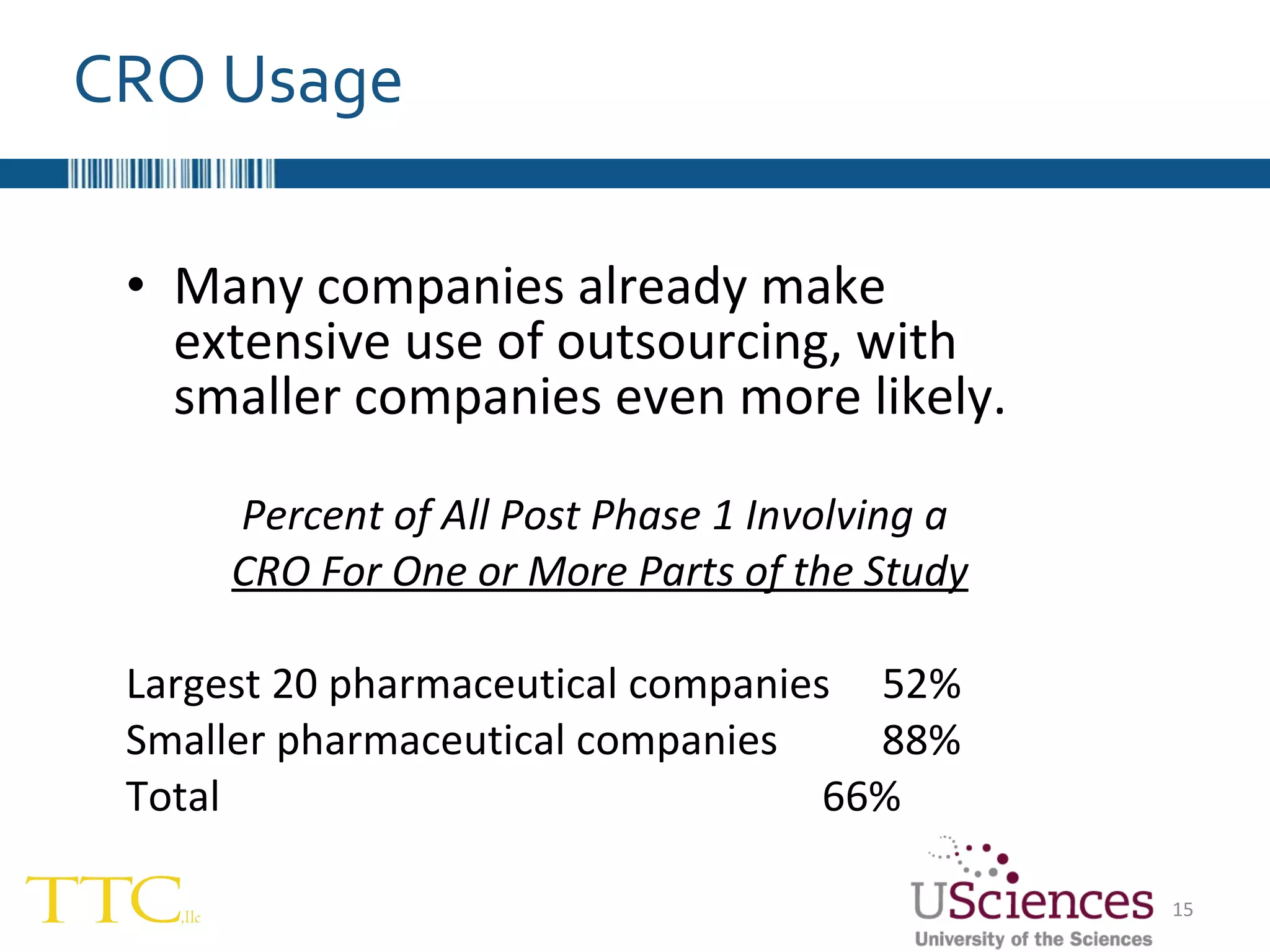
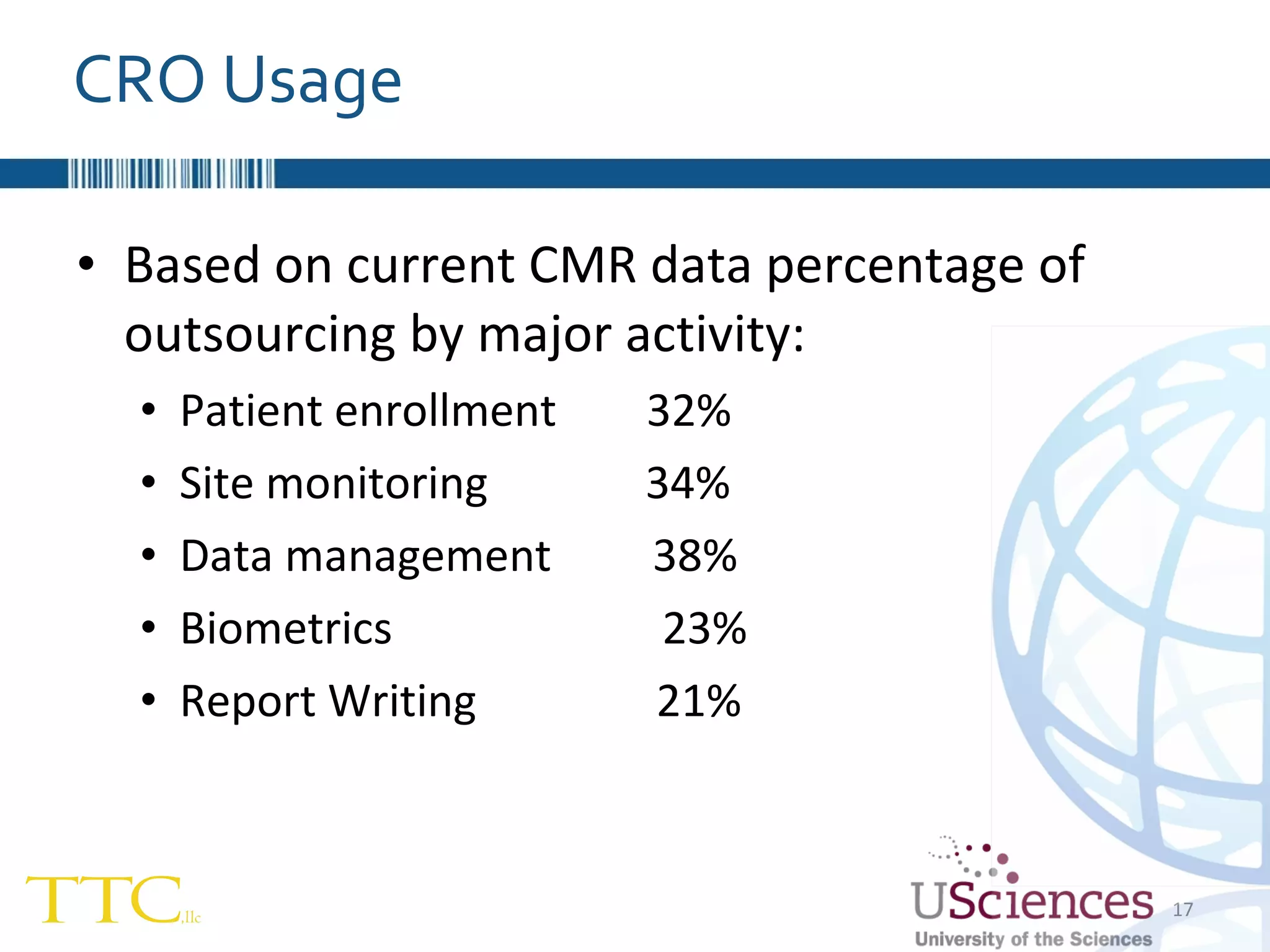
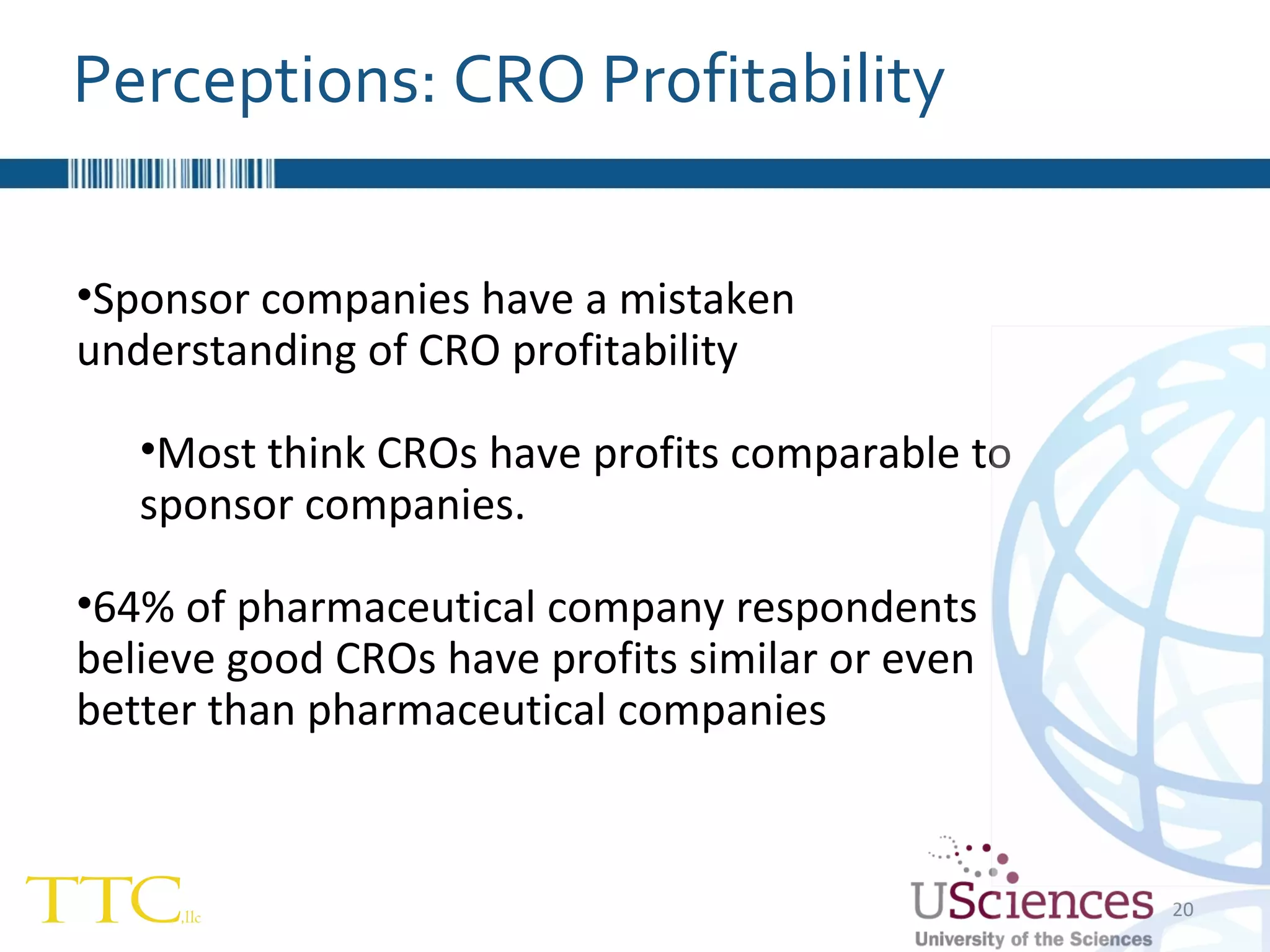
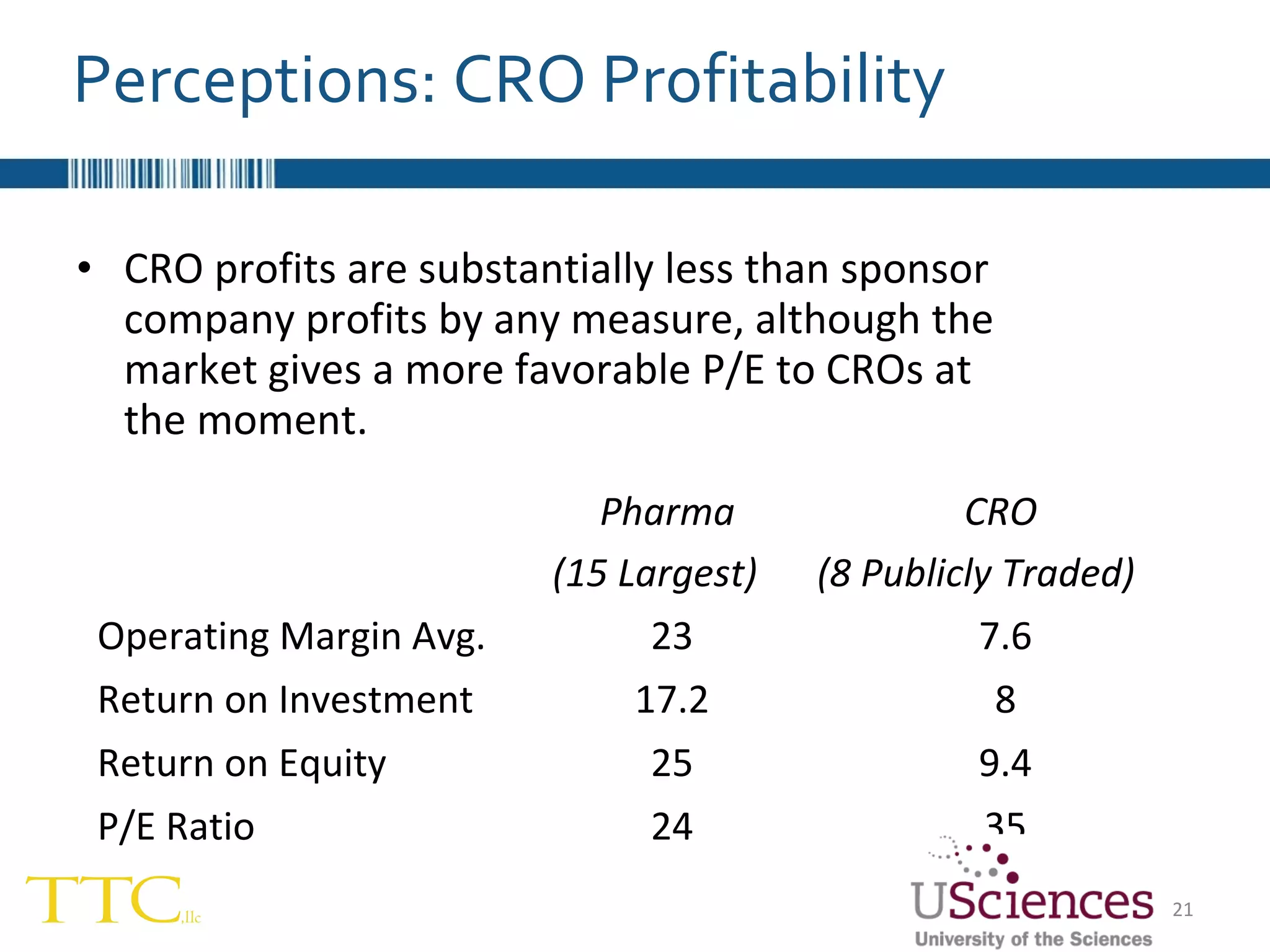
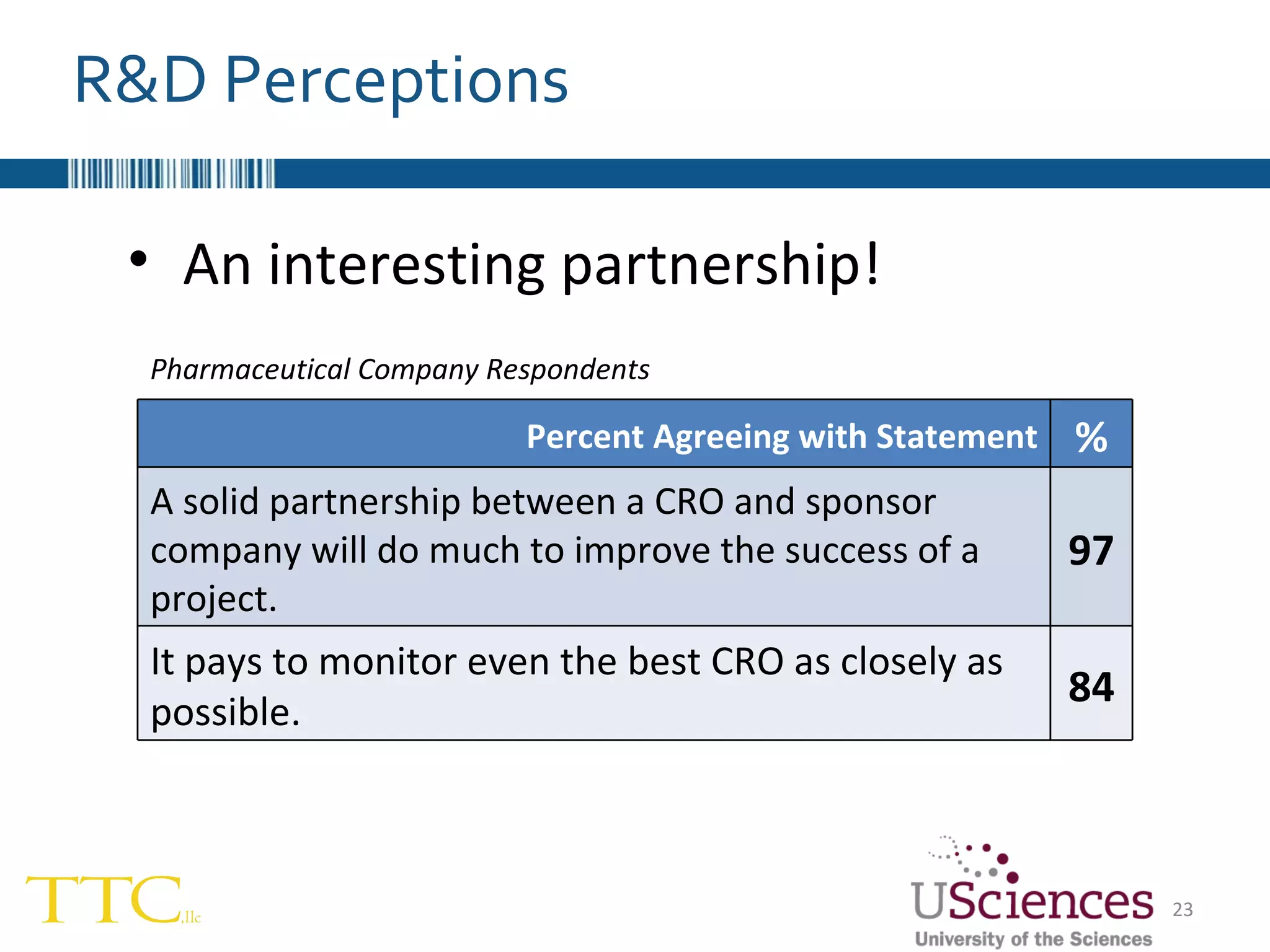
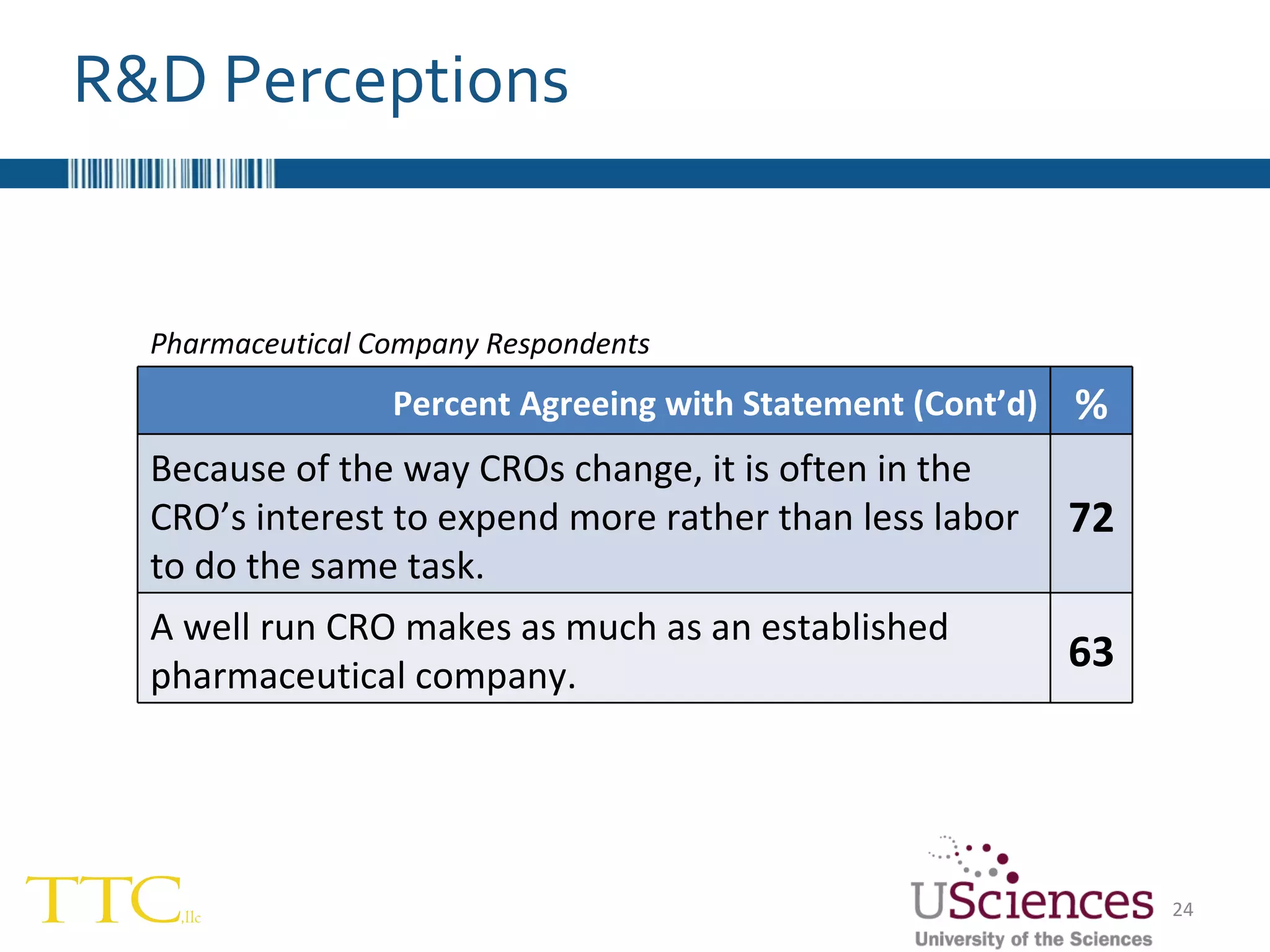
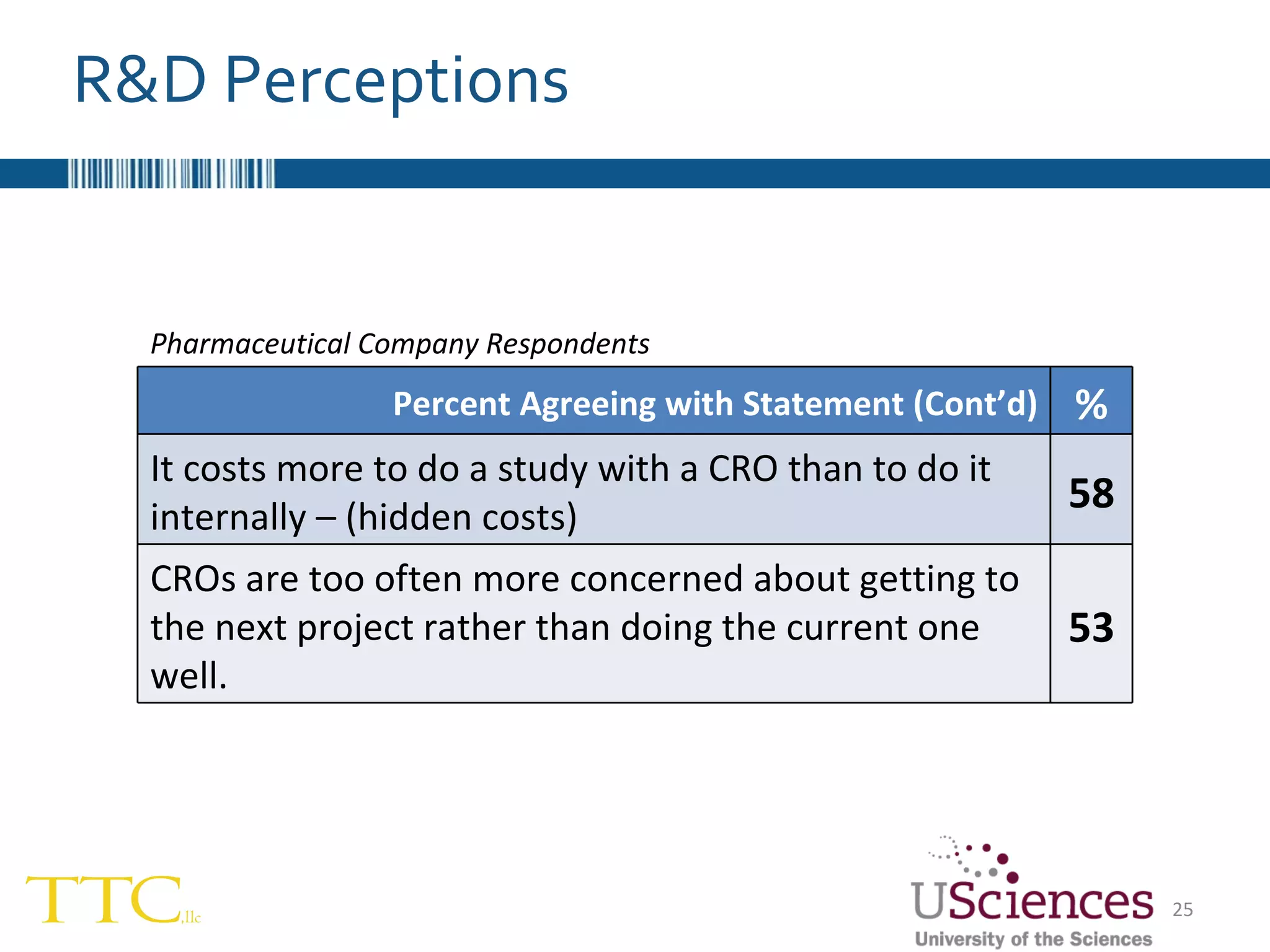
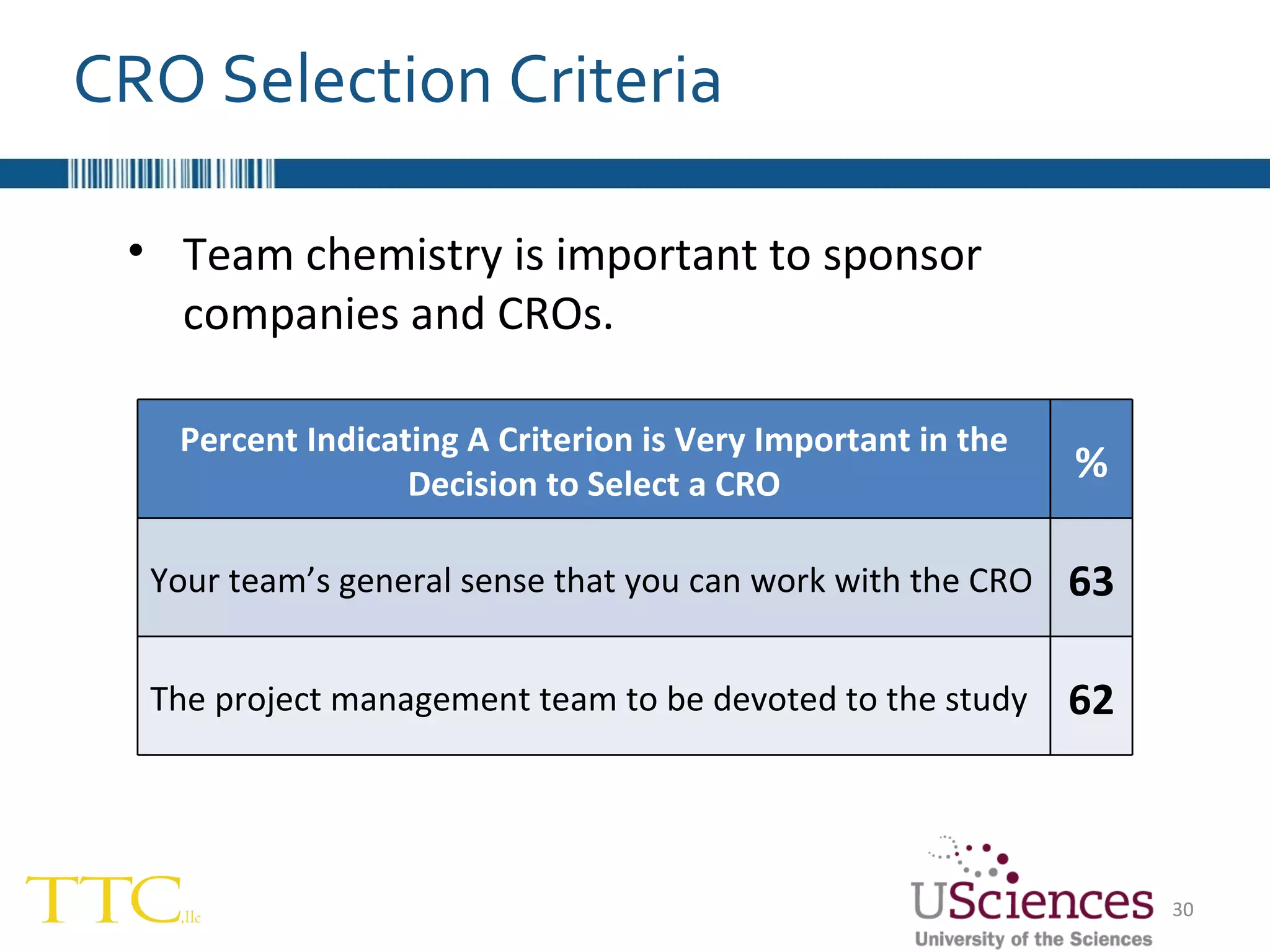
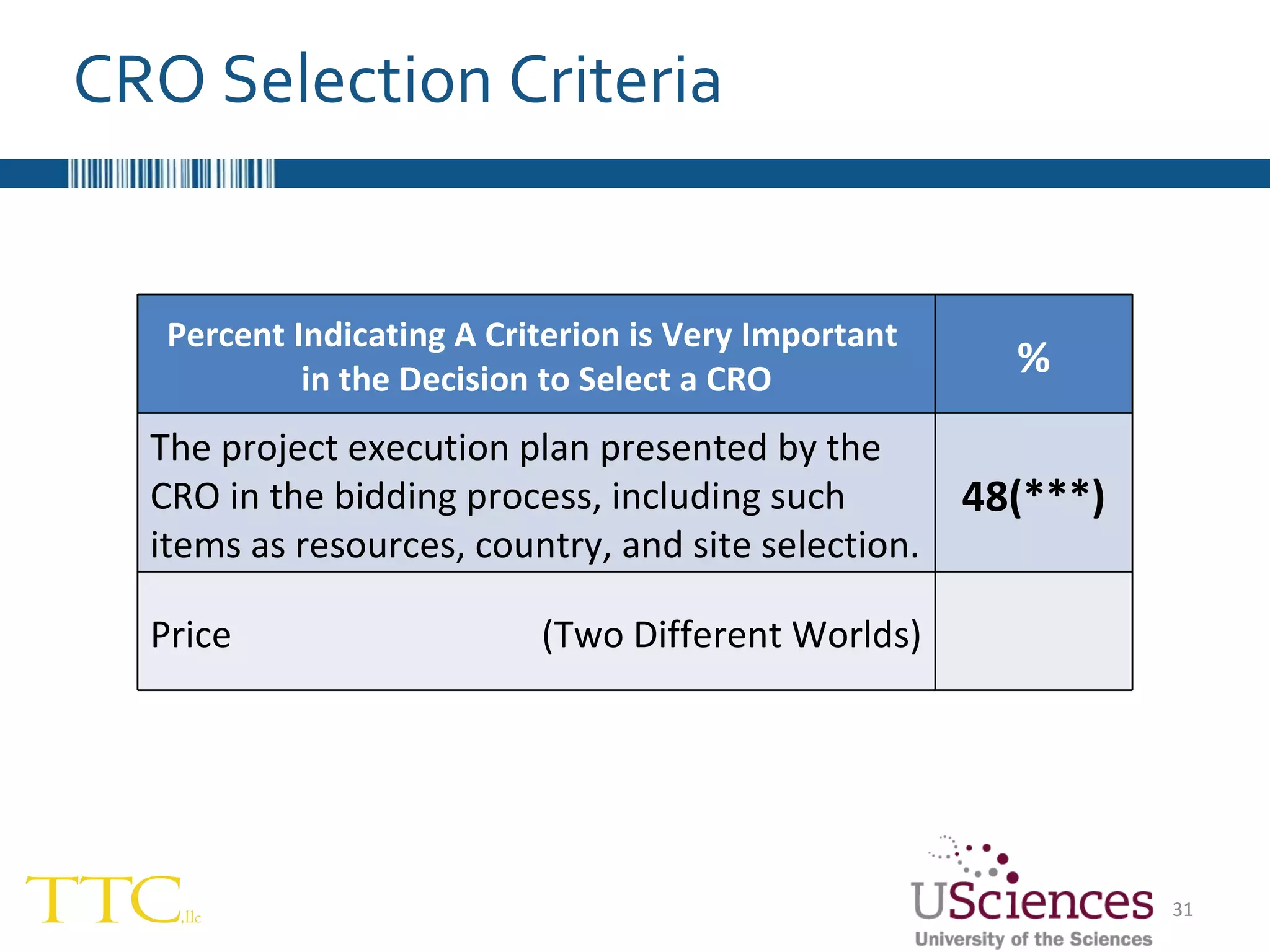
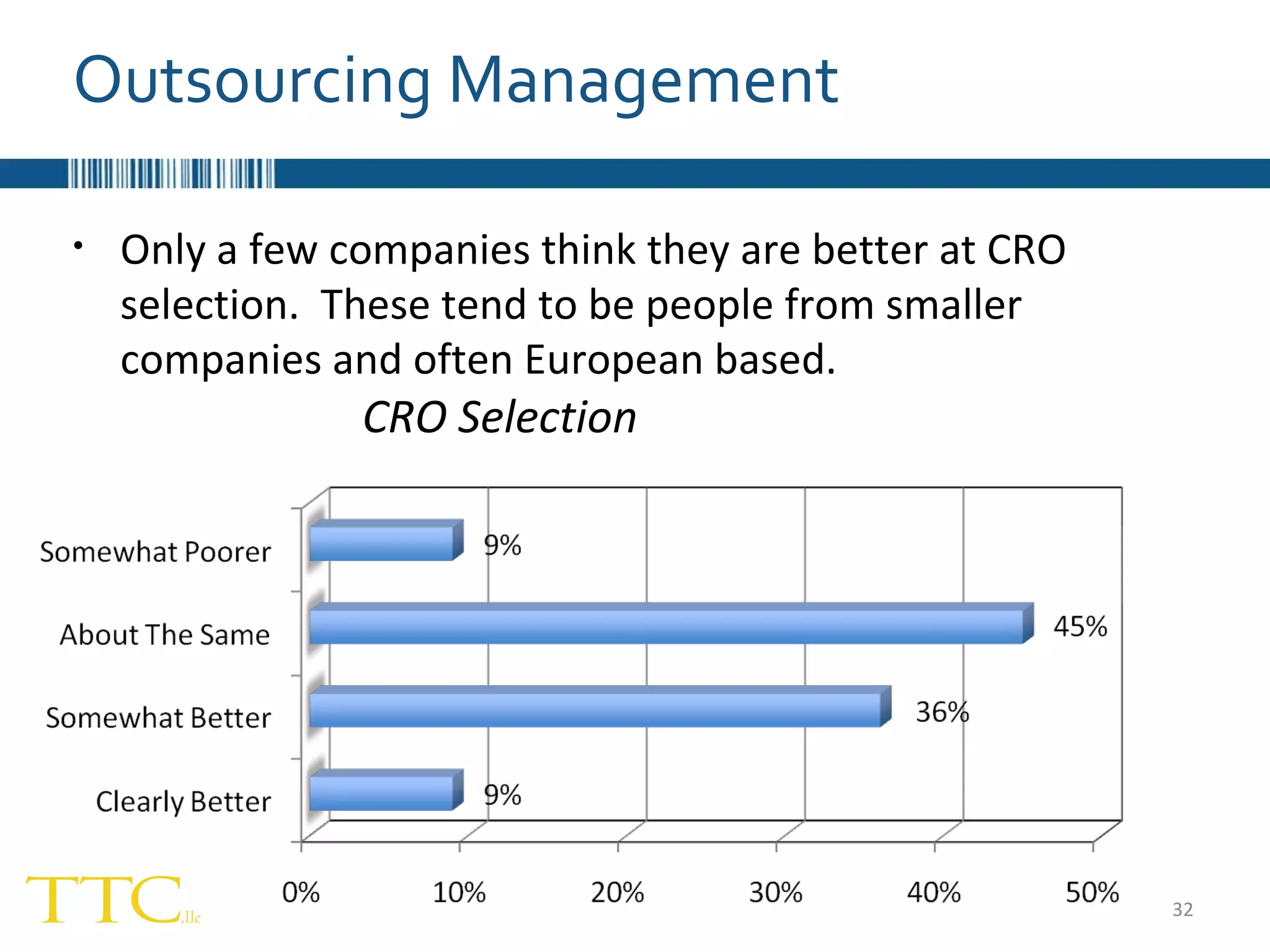
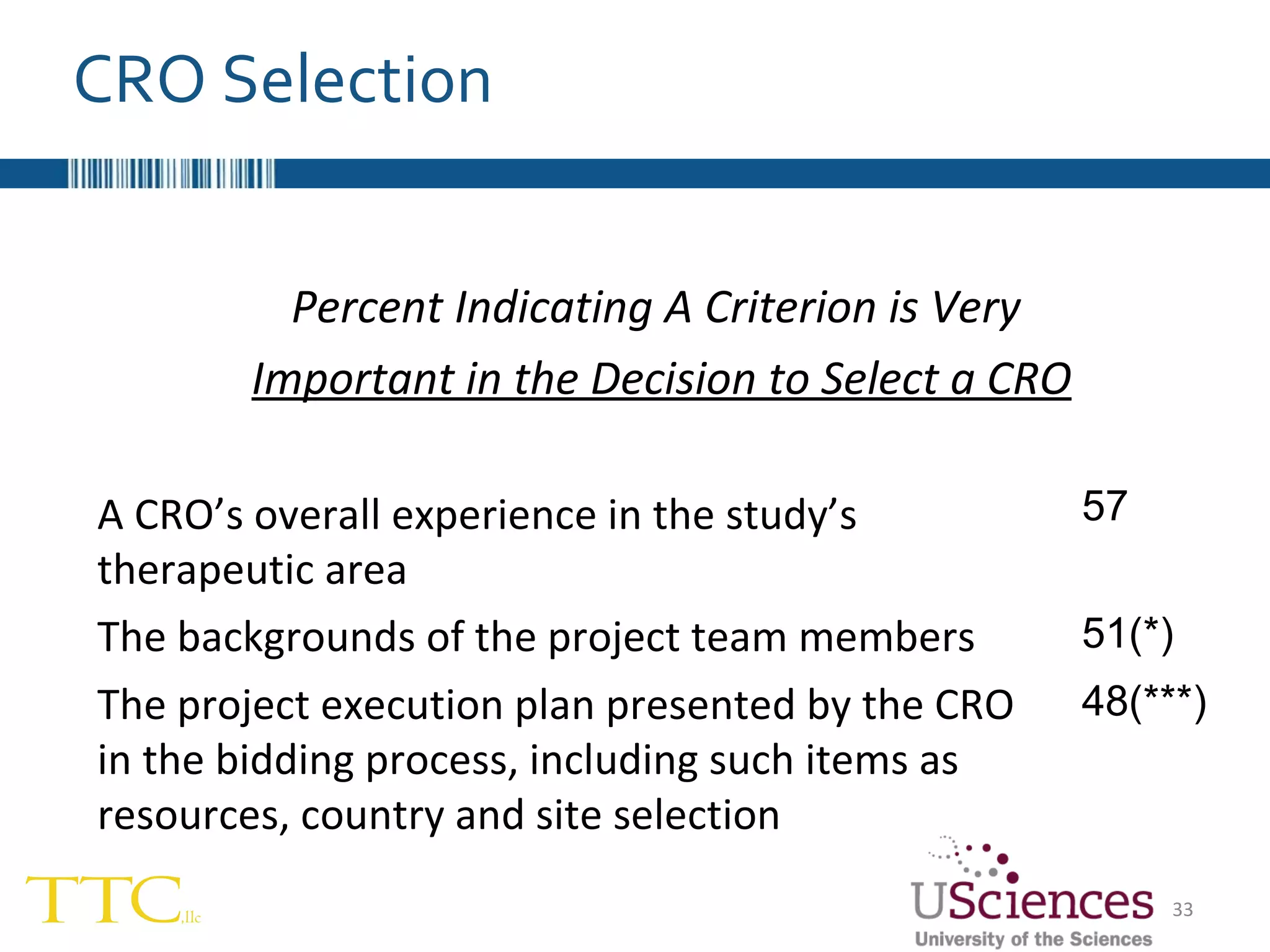
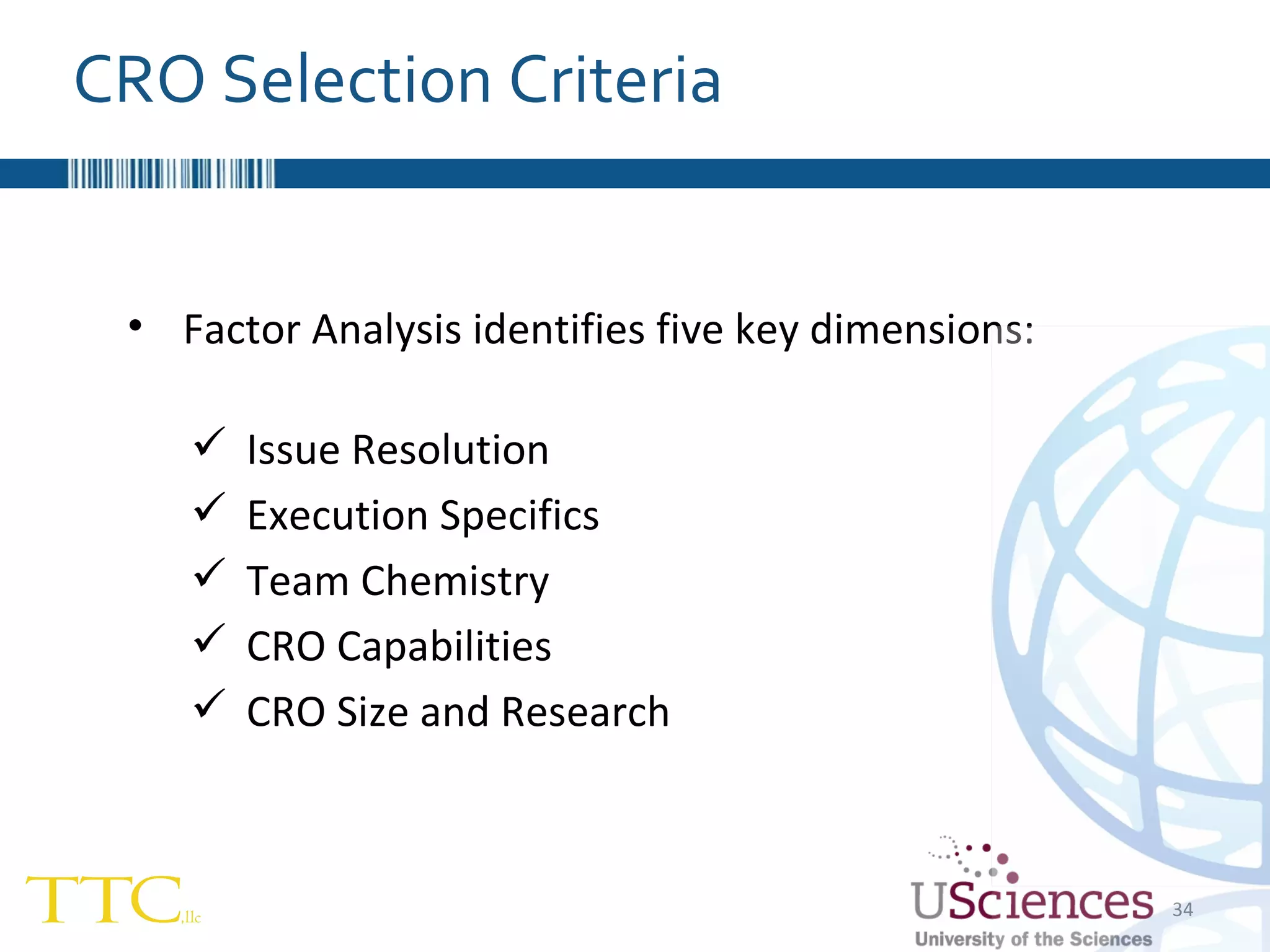
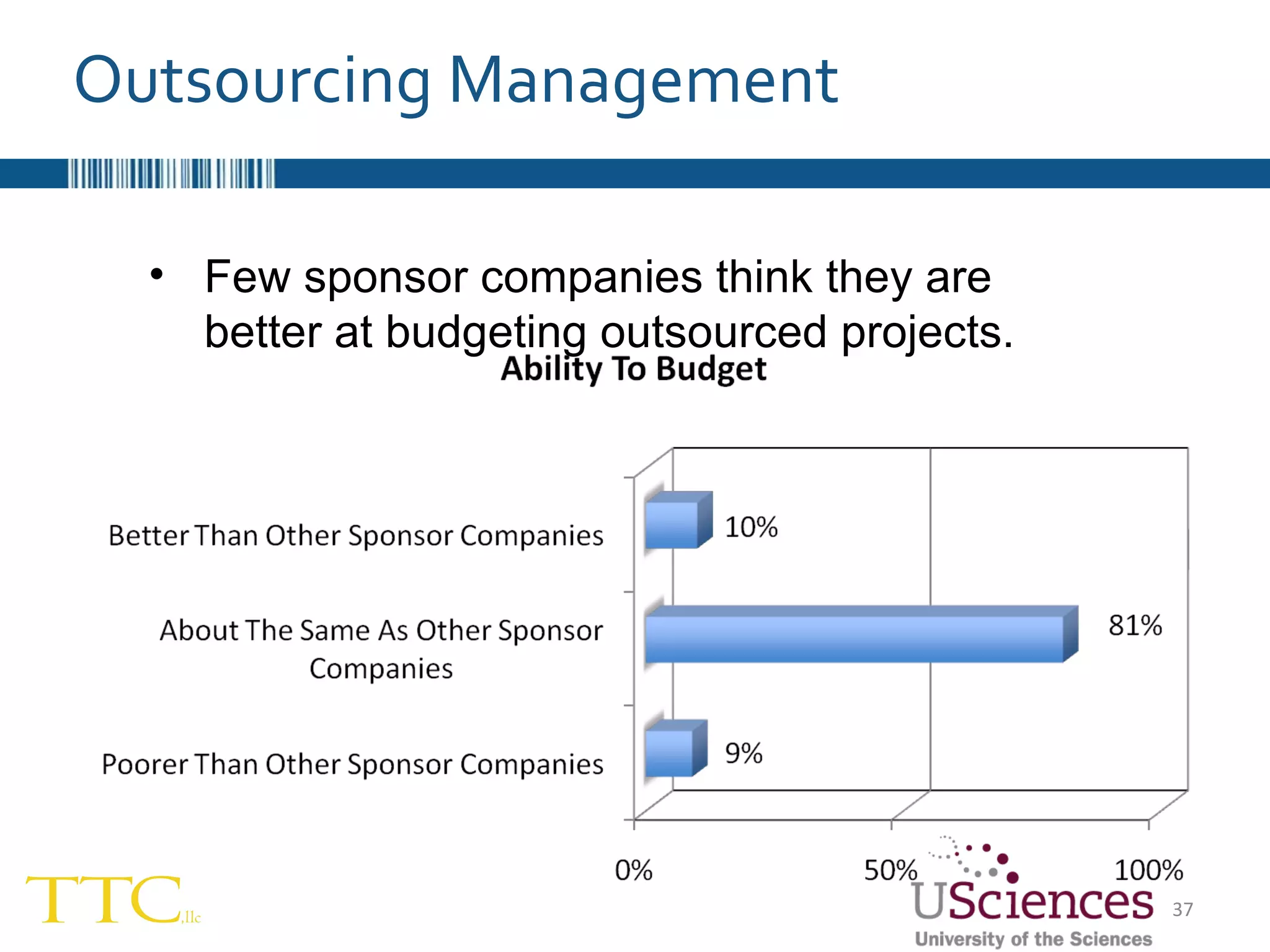
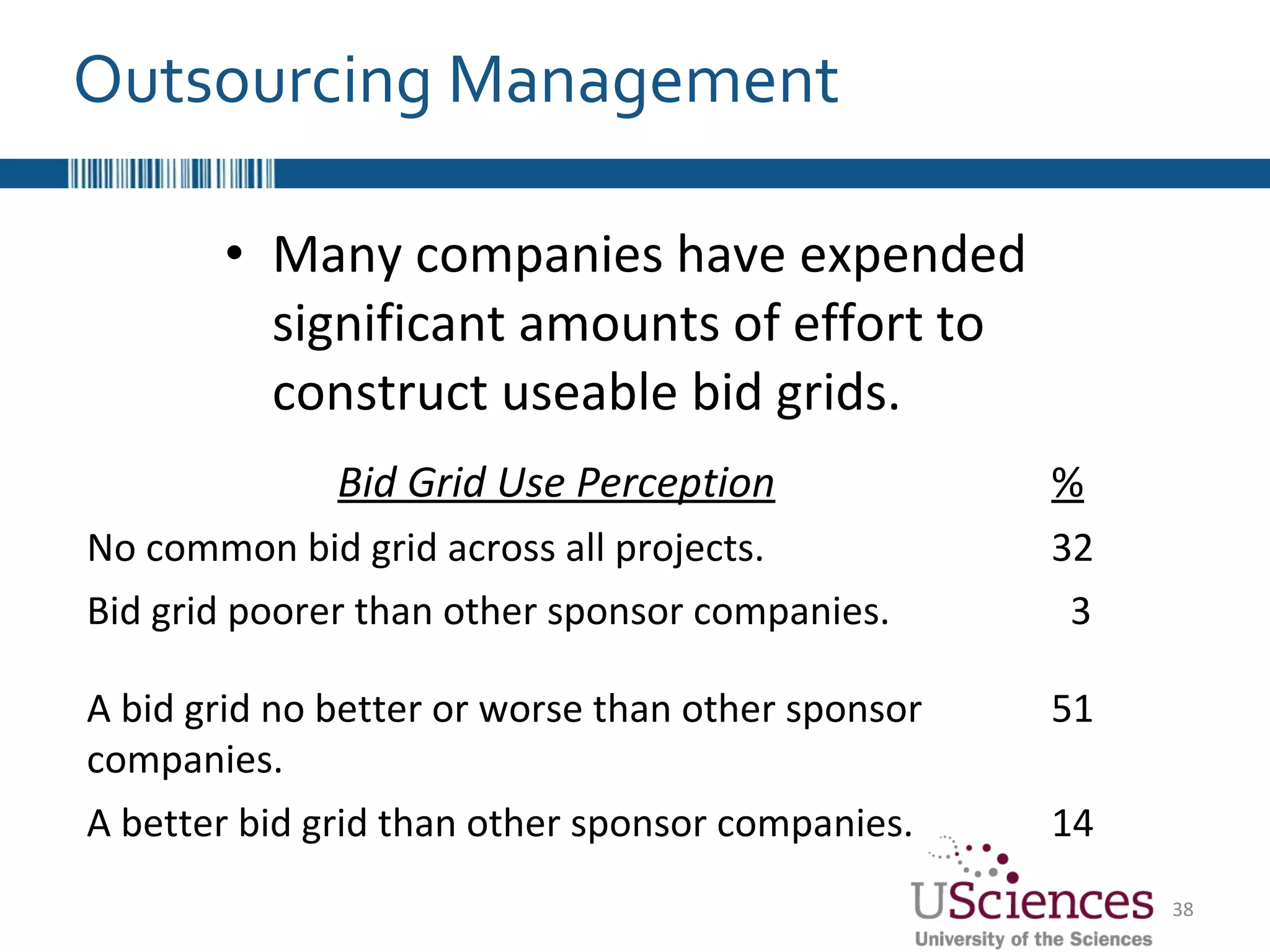
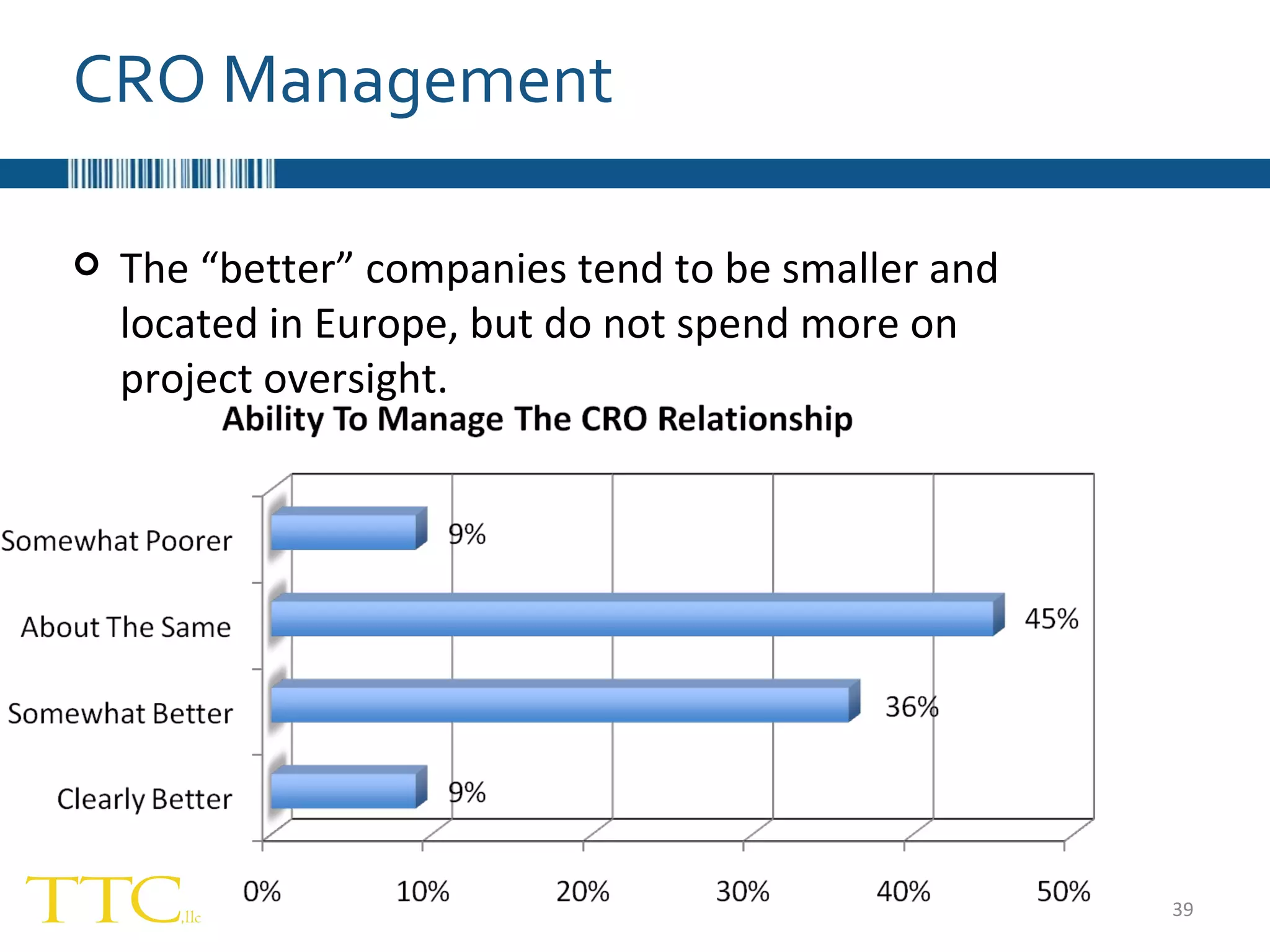
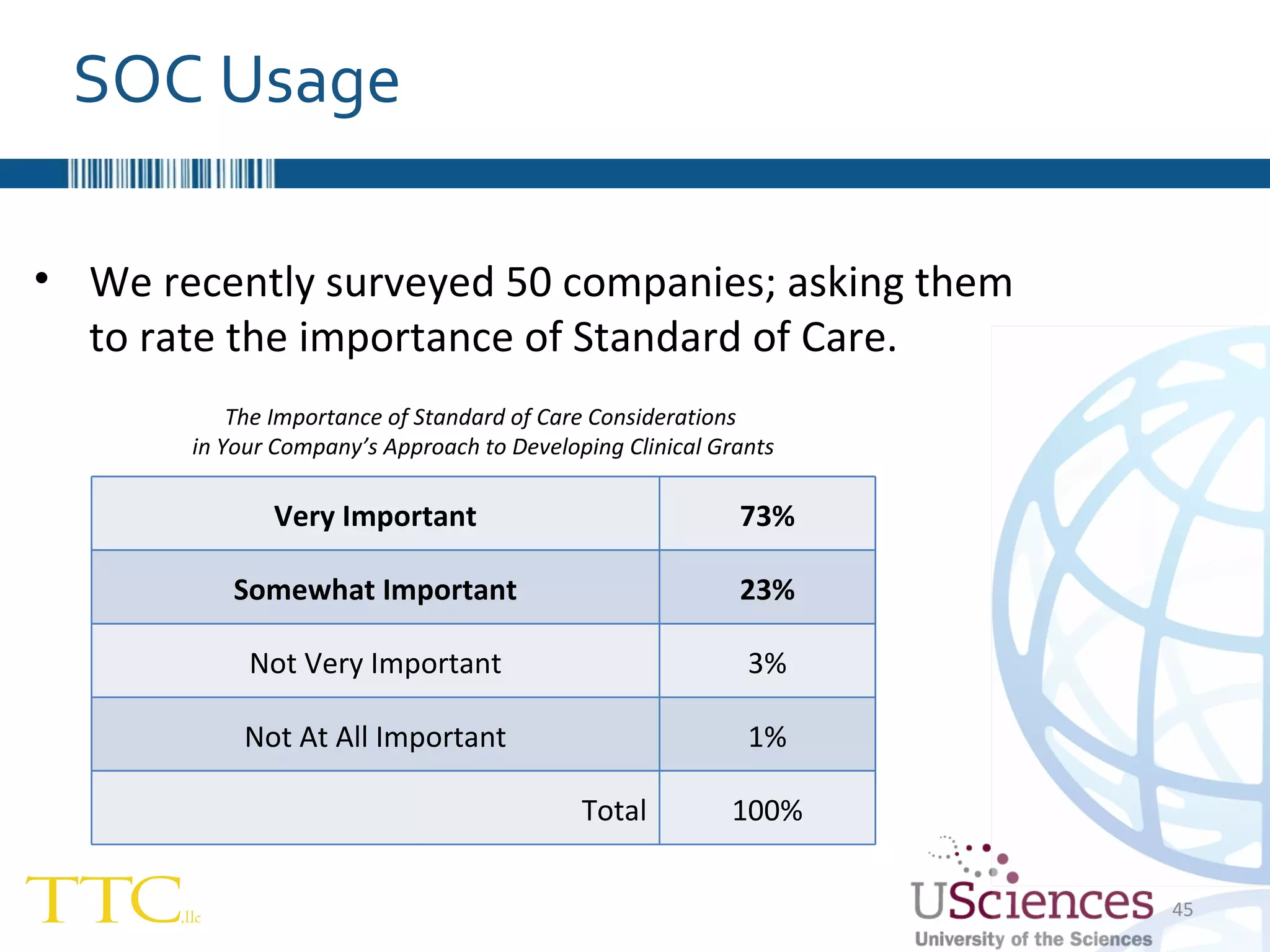
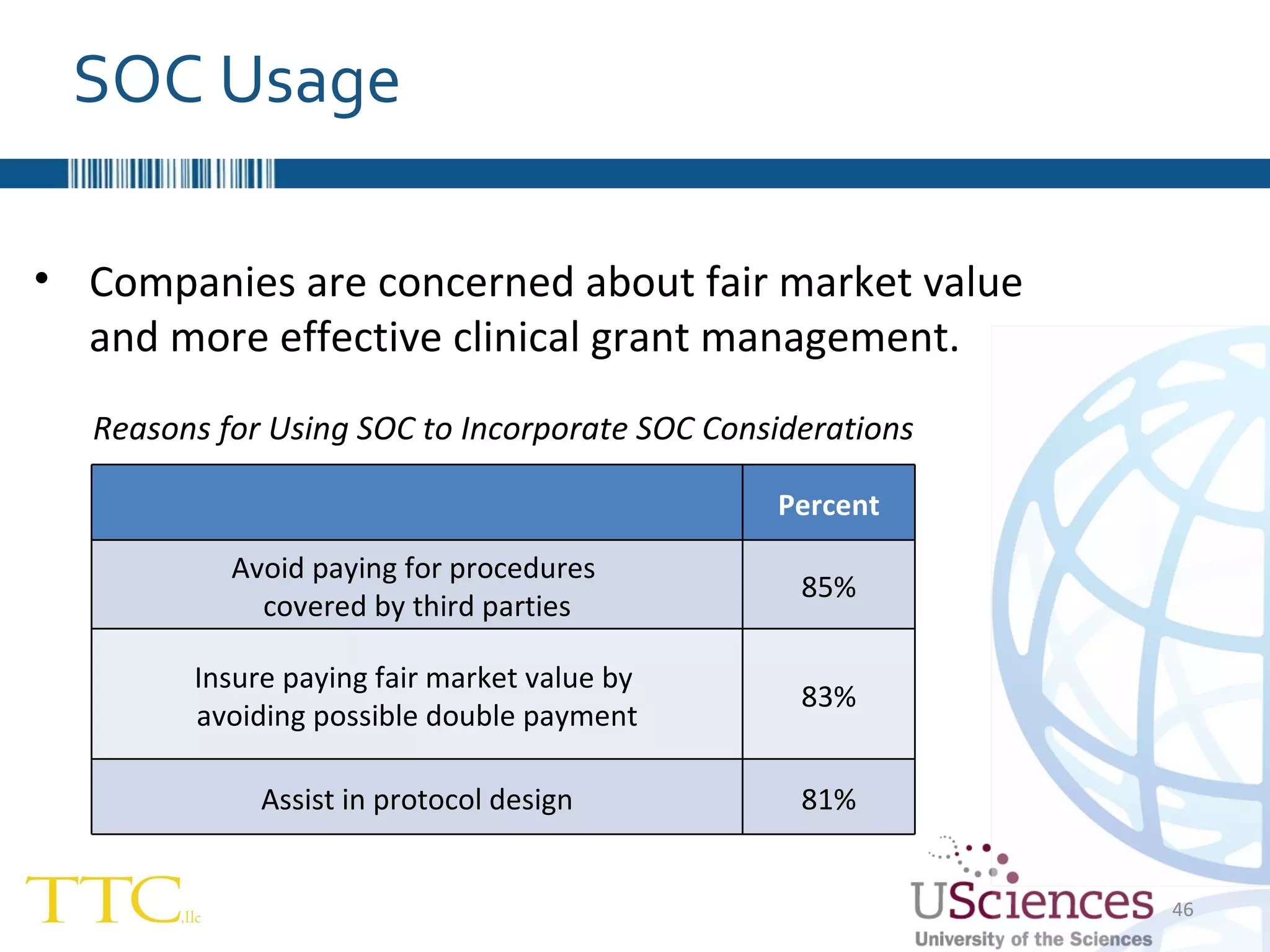
The document discusses the complex relationship between Contract Research Organizations (CROs) and pharmaceutical sponsors, highlighting their differing business models yet common goals. It outlines key issues in CRO selection, outsourcing management, and perceptions of profitability and effectiveness, underscoring the importance of collaboration and communication. Additionally, it addresses the implications of standard-of-care considerations in clinical trial funding, showing that many companies are concerned about fair market value and effective grant management.