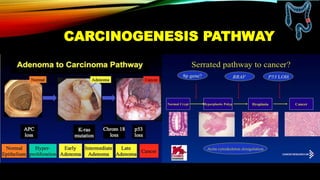
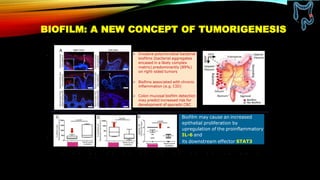
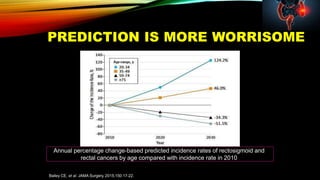
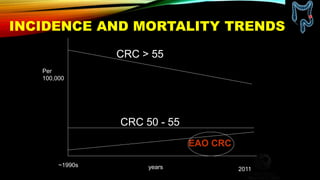
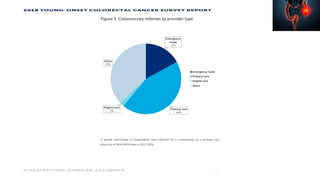
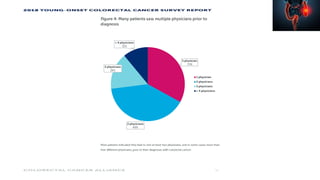
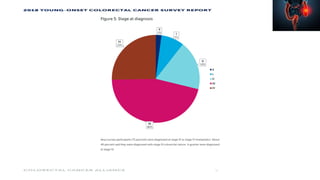
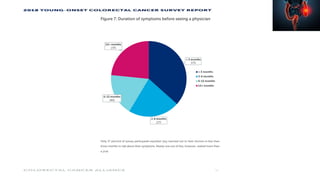
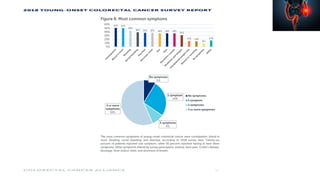
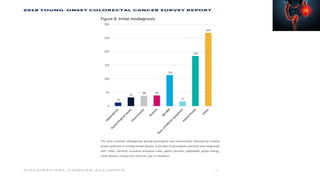
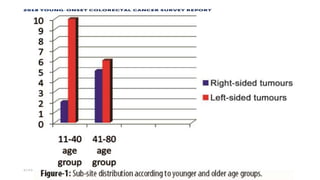
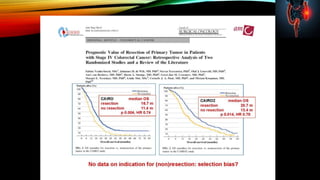
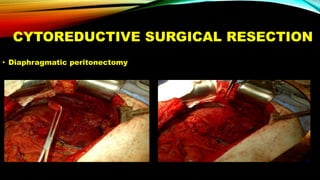
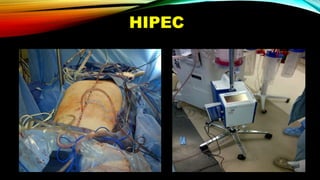
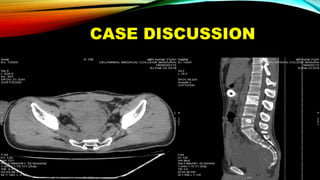
This document summarizes a tumor board discussion of a case involving a 21-year-old patient presenting with loose stools and abdominal pain for 2 months. Key details include KRAS mutation testing of the patient's tumor sample, which showed no mutation. The discussion covers epidemiology of early-onset colorectal cancer in India, treatment options including chemotherapy and targeted therapies, and factors influencing first-line treatment decisions for metastatic colorectal cancer such as biomarker status and primary tumor location.












![MADURAI 625009
TAMIL NADU INDIA
0452-2510000 8248883595
CLIENT CODE : C000051601
CLIENT'S NAME AND ADDRESS :
VELAMMAL MEDICAL COLLEGE HOSPITAL & RESEARCH INSTITUTE
VELLAMMAL VILLAGE, MADURAI-TUTICORIAN RING ROAF, ANUPPANADI,
DIAGNOSTIC REPORT
SRL LIMITED
PRIME SQUARE BUILDING,PLOT NO 1,GAIWADI INDUSTRIAL
ESTATE,S.V. ROAD,GOREGAON (W)
MUMBAI, 400062
MAHARASHTRA, INDIA
Tel : 1-800-222-000, Fax : 022 - 67801212
CIN - U74899PB1995PLC045956
Email : connect@srl.in
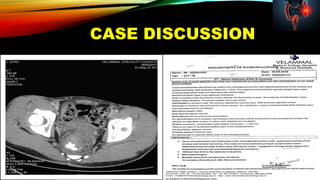
PATIENT ID :PATIENT NAME : AJITH KUMAR
ACCESSION NO : 0002RJ073694 AGE : 21 Years SEX : Male DATE OF BIRTH :
DRAWN : 27/10/2018 16:00 RECEIVED : 29/10/2018 07:12 REPORTED : 03/11/2018 15:39
REFERRING DOCTOR : CLIENT PATIENT ID : 1805030173
Final ResultsTest Report Status Units
KRAS MUTATION DETECTION
KRAS MUTATION AT CODON 12 (EXON 2)
NOT DETECTED
DETECTED
KRAS MUTATION AT CODON 13 (EXON 2)
NOT DETECTED
DETECTED
BLOCK IDENTIFICATION NUMBER S-3175/18-A10
Interpretation(s)
KRAS MUTATION DETECTION-
Background: Somatic mutations of KRAS gene represent the most common alterations currently known in colon rectal cancer (CRC). In addition, subsets of NSCLC patients
also harbour KRAS mutation especially who have EGFR widltype genotype. KRAS mutation is found to be reported in 30-45 % of CRC patients, has shown to be a predictive
biomarker of resistance to anti-EGFR antibody therapy [Loupakis F et al 2009 Soeda H et al 2013].
Clinical utility: KRAS codons 12 and 13 mutations predict resistance to anti-EGFR monoclonal antibodies in metastatic colorectal cancer.
Test method: Pyrosequencing.
Result interpretation:
A)Positive: The result indicates that the patient’s tumor sample shows either KRAS codon 12 or codon 13 mutation. Presence of KRAS mutation in codon 12 or 13 is useful in
identifying patients who are likely to show resistance to anti-EGFR therapy in metastatic colorectal cancer. Based on American Society of Clinical Oncology Provisional Clinical
Opinion, treatment with anti-EGFR monoclonal antibody therapy is not recommended for patients harboring codon 12 or 13 mutation in the KRASgene.
B) Negative: The result indicates that the patient’s tumor sample do not show KRAS codon 12 and 13 mutation.
Limitations:
PCR is highly sensitive technique however inherent PCR inhibitors in the specimen may result into amplification failure.
Pyrosequencing has a sensitivity of 10% mutant allele. Tissues with less than 20% of tumor cells may not be detect mutation in KRAS codon 12 and 13.
The test will detect most common mutations in codon 12 and 13 of the KRAS gene. Mutations in other locations within the KRAS gene will not be detected.
References:
Loupakis F1, Ruzzo A, Cremolini C et al (2009). KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type
metastatic colorectal cancer. Br J Cancer. Aug 18 101(4):715-21.
Soeda H, Shimodaira H, Watanabe M, et al (2013). Clinical usefulness of KRAS, BRAF, and PIK3CA mutations as predictive markers of cetuximab efficacy in irinotecan- and
oxaliplatin-refractory Japanese patients with metastatic colorectal cancer. Int J Clin Oncol. Aug 18(4):670-7.
**End Of Report**
Please visit www.srlworld.com for related Test Information for this accession
Dr. Firoz Ahmad,PhD
Research Scientist and Senior
Manager - R&D
Dr. B. R. Das, PhD
Advisor and Mentor
R&D & Molecular Diagnostics
Dr. (COL) Prabal Deb
Director (Lab Operations) &
Chief Histopathologist
Page 1 Of 2](https://image.slidesharecdn.com/tumorboard-200905154815/85/Tumor-board-24-320.jpg)


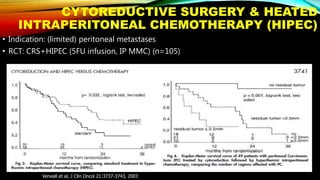
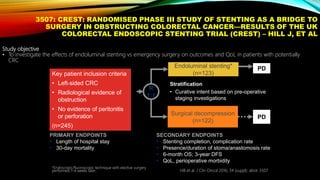
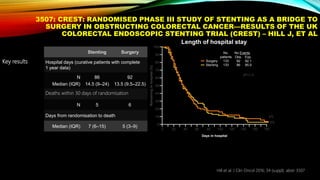







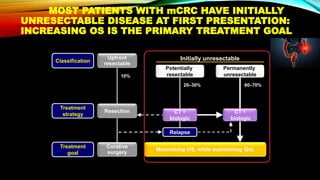
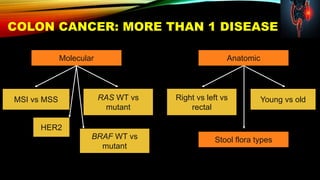

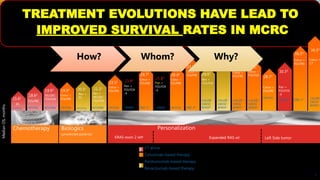
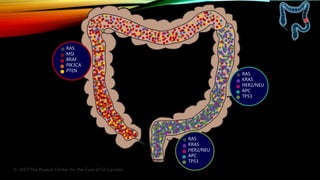

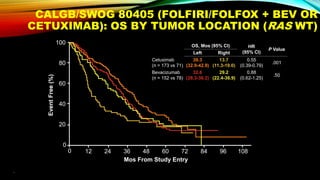
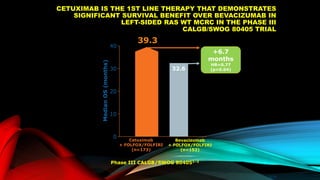
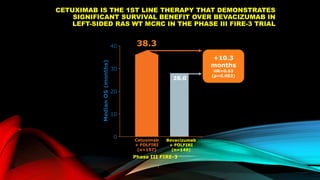
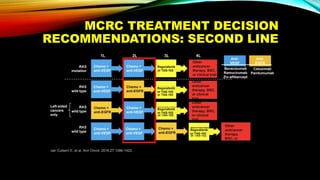
![SEVERAL FACTORS INFLUENCE 1ST LINE
TREATMENT DECISIONS IN MCRC
1. Van Cutsem E, et al. Ann Oncol 2016;27:1386–1422; 2. Lenz H-J, et al. Ann Oncol 2014;25 (suppl 4; Abstract No. 501O); 3. Stintzing S, et al.
Lancet 2016;17:1426–1434; 4. Arnold D, et al. Ann Oncol 2017; doi: 10.1093; 5. Heinemann V, et al. Lancet Oncol 2014;15:1065–1075; 6.
Venook A, et al. JAMA 2017;317:2392–2401.
*FIRE-3 did not meet its primary endpoint of significantly improving overall response rate (ORR) based on investigators’ read in
patients with KRAS (exon 2) wt mCRC;5 †CALGB/SWOG 80405 study did not meet its primary endpoint of significantly
improving overall survival in the cetuximab + CT arm vs bevacizumab + CT arm in patients with KRAS (exon 2) wt mCRC.6
CT, chemotherapy
• Including BRAF, MSI and RAS status1
• RAS status predicts outcomes with
anti-EGFR therapies1
• In RAS wt mCRC, cetuximab + CT is
associated with greater
improvements in ORR, OS and PFS
over bevacizumab + CT2,3*† [CALGB
SWOG/80405, FIRE-3]
Biomarker status1–3
Patient characteristics1
Primary tumor location4](https://image.slidesharecdn.com/tumorboard-200905154815/85/Tumor-board-48-320.jpg)